Stress-induced gene expression requires programmed recovery from translational repression
- PMID: 12606582
- PMCID: PMC150345
- DOI: 10.1093/emboj/cdg112
Stress-induced gene expression requires programmed recovery from translational repression
Erratum in
- EMBO J. 2003 May 1;22(9):2307
Abstract
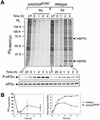
Active repression of protein synthesis protects cells against protein malfolding during endoplasmic reticulum stress, nutrient deprivation and oxidative stress. However, long-term adaptation to these conditions requires synthesis of new stress-induced proteins. Phosphorylation of the alpha-subunit of translation initiation factor 2 (eIF2alpha) represses translation in diverse stressful conditions. GADD34 is a stress-inducible regulatory subunit of a holophosphatase complex that dephosphorylates eIF2alpha, and has been hypothesized to play a role in translational recovery. Here, we report that GADD34 expression correlated temporally with eIF2alpha dephosphorylation late in the stress response. Inactivation of both alleles of GADD34 prevented eIF2alpha dephosphorylation and blocked the recovery of protein synthesis, normally observed late in the stress response. Furthermore, defective recovery of protein synthesis markedly impaired translation of stress-induced proteins and interfered with programmed activation of stress-induced genes in the GADD34 mutant cells. These observations indicate that GADD34 controls a programmed shift from translational repression to stress-induced gene expression, and reconciles the apparent contradiction between the translational and transcriptional arms of cellular stress responses.
Figures





References
-
- Brostrom C.O. and Brostrom,M.A. (1998) Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol., 58, 79–125. - PubMed
-
- Brostrom M.A., Lin,X.J., Cade,C., Gmitter,D. and Brostrom,C.O. (1989) Loss of a calcium requirement for protein synthesis in pituitary cells following thermal or chemical stress. J. Biol. Chem., 264, 1638–1643. - PubMed
-
- Calfon M., Zeng,H., Urano,F., Till,J.H., Hubbard,S.R., Harding,H.P., Clark,S.G. and Ron,D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature, 415, 92–96. - PubMed
-
- DeGracia D.J., Kumar,R., Owen,C.R., Krause,G.S. and White,B.C. (2002) Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. J. Cereb. Blood Flow Metab., 22, 127–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

