Searching for mechanisms of N-methyl-D-aspartate-induced glutathione efflux in organotypic hippocampal cultures
- PMID: 12608701
- PMCID: PMC1475825
- DOI: 10.1023/a:1022381318126
Searching for mechanisms of N-methyl-D-aspartate-induced glutathione efflux in organotypic hippocampal cultures
Abstract
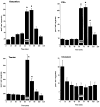
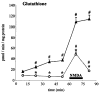
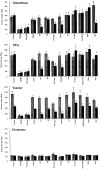
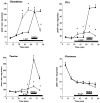
N-Methyl-D-aspartate (NMDA)-receptor stimulation evoked a selective and partly delayed elevated efflux of glutathione, phosphoethanolamine, and taurine from organotypic rat hippocampus slice cultures. The protein kinase inhibitors H9 and staurosporine had no effect on the efflux. The phospholipase A2 inhibitors quinacrine and 4-bromophenacyl bromide, as well as arachidonic acid, a product of phospholipase A2 activity, did not affect the stimulated efflux. Polymyxin B, an antimicrobal agent that inhibits protein kinase C, and quinacrine in high concentration (500 microM), blocked efflux completely. The stimulated efflux after but not during NMDA incubation was attenuated by a calmodulin antagonist (W7) and an anion transport inhibitor (DNDS). Omission of calcium increased the spontaneous efflux with no or small additional effects by NMDA. In conclusion, NMDA receptor stimulation cause an increased selective efflux of glutathione, phosphoethanolamine and taurine in organotypic cultures of rat hippocampus. The efflux may partly be regulated by calmodulin and DNDS sensitive channels.
Figures
Similar articles
-
NMDA-receptor mediated efflux of N-acetylaspartate: physiological and/or pathological importance?Neurochem Int. 2004 Dec;45(8):1195-204. doi: 10.1016/j.neuint.2004.06.005. Neurochem Int. 2004. PMID: 15380629
-
Glutathione efflux induced by NMDA and kainate: implications in neurotoxicity?J Neurochem. 1999 Oct;73(4):1566-72. doi: 10.1046/j.1471-4159.1999.0731566.x. J Neurochem. 1999. PMID: 10501202
-
Exposure to N-methyl-D-aspartate increases release of arachidonic acid in primary cultures of rat hippocampal neurons and not in astrocytes.Brain Res. 1990 Sep 3;526(2):241-8. doi: 10.1016/0006-8993(90)91228-9. Brain Res. 1990. PMID: 2124161
-
Taurine release evoked by NMDA receptor activation is largely dependent on calcium mobilization from intracellular stores.Eur J Neurosci. 1993 Oct 1;5(10):1273-9. doi: 10.1111/j.1460-9568.1993.tb00912.x. Eur J Neurosci. 1993. PMID: 8275229
-
Regulatory role of nitric oxide over extracellular taurine in the hippocampus of freely moving rats.Neurosci Lett. 2004 Mar 11;357(3):179-82. doi: 10.1016/j.neulet.2003.12.056. Neurosci Lett. 2004. PMID: 15003279
Cited by
-
Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins.Antioxidants (Basel). 2021 Jan 11;10(1):89. doi: 10.3390/antiox10010089. Antioxidants (Basel). 2021. PMID: 33440661 Free PMC article. Review.
-
Polymyxin B enhances acrosomal exocytosis triggered by calcium and the calcium ionophore A23187 in ejaculated boar spermatozoa.Anim Sci J. 2019 Jun;90(6):705-711. doi: 10.1111/asj.13155. Epub 2019 Apr 11. Anim Sci J. 2019. PMID: 30972868 Free PMC article.
-
Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels.J Biol Chem. 2008 Apr 18;283(16):10347-56. doi: 10.1074/jbc.M704153200. Epub 2008 Feb 13. J Biol Chem. 2008. PMID: 18272524 Free PMC article.
References
-
- Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. - PubMed
-
- Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-d-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. - PubMed
-
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. - PubMed
-
- Hagberg H, Lehmann A, Sandberg M, Nyström B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab. 1985;5:413–419. - PubMed
-
- Kawaguchi K, Huerbin M, Simon RP. Lesioning of deep prepiriform cortex protects against ischemic neuronal necrosis by attenuating extracellular glutamate concentrations. J Neurochem. 1997;69:412–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
