A simple kinetic model describes the processivity of myosin-v
- PMID: 12609867
- PMCID: PMC1302734
- DOI: 10.1016/S0006-3495(03)74973-X
A simple kinetic model describes the processivity of myosin-v
Abstract
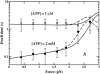
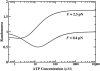
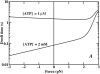
Myosin-V is a motor protein responsible for organelle and vesicle transport in cells. Recent single-molecule experiments have shown that it is an efficient processive motor that walks along actin filaments taking steps of mean size close to 36 nm. A theoretical study of myosin-V motility is presented following an approach used successfully to analyze the dynamics of conventional kinesin but also taking some account of step-size variations. Much of the present experimental data for myosin-V can be well described by a two-state chemical kinetic model with three load-dependent rates. In addition, the analysis predicts the variation of the mean velocity and of the randomness-a quantitative measure of the stochastic deviations from uniform, constant-speed motion-with ATP concentration under both resisting and assisting loads, and indicates a substep of size d(0) approximately 13-14 nm (from the ATP-binding state) that appears to accord with independent observations.
Figures
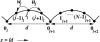



Similar articles
-
Dynamics of myosin-V processivity.Biophys J. 2005 Feb;88(2):999-1008. doi: 10.1529/biophysj.104.047662. Epub 2004 Nov 19. Biophys J. 2005. PMID: 15556991 Free PMC article.
-
Characteristics of Myosin V Processivity.IEEE/ACM Trans Comput Biol Bioinform. 2019 Jul-Aug;16(4):1302-1308. doi: 10.1109/TCBB.2017.2669311. Epub 2017 Feb 14. IEEE/ACM Trans Comput Biol Bioinform. 2019. PMID: 28212094
-
Understanding mechanochemical coupling in kinesins using first-passage-time processes.Phys Rev E Stat Nonlin Soft Matter Phys. 2005 Mar;71(3 Pt 1):031902. doi: 10.1103/PhysRevE.71.031902. Epub 2005 Mar 8. Phys Rev E Stat Nonlin Soft Matter Phys. 2005. PMID: 15903454
-
Molecular motors: a theorist's perspective.Annu Rev Phys Chem. 2007;58:675-95. doi: 10.1146/annurev.physchem.58.032806.104532. Annu Rev Phys Chem. 2007. PMID: 17163836 Review.
-
The role of thermal activation in motion and force generation by molecular motors.Philos Trans R Soc Lond B Biol Sci. 2000 Apr 29;355(1396):511-22. doi: 10.1098/rstb.2000.0592. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10836504 Free PMC article. Review.
Cited by
-
A Unified Walking Model for Dimeric Motor Proteins.Biophys J. 2018 Nov 20;115(10):1981-1992. doi: 10.1016/j.bpj.2018.09.032. Epub 2018 Oct 16. Biophys J. 2018. PMID: 30396511 Free PMC article.
-
Statistical kinetics of macromolecular dynamics.Biophys J. 2005 Oct;89(4):2277-85. doi: 10.1529/biophysj.105.064295. Epub 2005 Jul 22. Biophys J. 2005. PMID: 16040752 Free PMC article.
-
Kinetic models of redox-coupled proton pumping.Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2169-74. doi: 10.1073/pnas.0611114104. Epub 2007 Feb 7. Proc Natl Acad Sci U S A. 2007. PMID: 17287344 Free PMC article.
-
Dynamics of ATP-dependent and ATP-independent steppings of myosin-V on actin: catch-bond characteristics.J R Soc Interface. 2020 Apr;17(165):20200029. doi: 10.1098/rsif.2020.0029. Epub 2020 Apr 8. J R Soc Interface. 2020. PMID: 32259459 Free PMC article.
-
Kinetic gating of the proton pump in cytochrome c oxidase.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13707-12. doi: 10.1073/pnas.0903938106. Epub 2009 Aug 3. Proc Natl Acad Sci U S A. 2009. PMID: 19666617 Free PMC article.
References
-
- Block, S. M., L. S. B. Goldstein, and B. J. Schnapp. 1990. Bead movements by single kinesin molecules studied with optical tweezers. Nature. 348:348–352. - PubMed
-
- Block, S. M. 2001. Molecular Motors (Lecture). 55th Annual Meeting of the Society of General Physiologists, Sept. 9, 2001, Woods Hole, Massachusetts.
-
- Bray, D. 2001. Cell Movements: From Molecules to Motility, 2nd Ed. Garland Publishing, New York. Chapter 5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

