Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS
- PMID: 12609870
- PMCID: PMC1302737
- DOI: 10.1016/S0006-3495(03)74976-5
Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS
Abstract
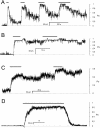
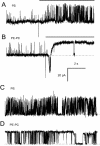
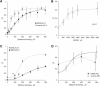
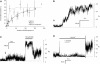
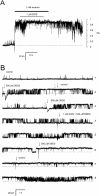
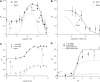
Activation of skeletal muscle ryanodine receptors (RyRs) by suramin and disulfonic stilbene derivatives (Diisothiocyanostilbene-2',2'-disulfonic acid (DIDS), 4,4'-dibenzamidostilbene-2,2'-disulfonic acid (DBDS),and 4,4'-dinitrostilbene-2,2'-disulfonic acid (DNDS)) was investigated using planar bilayers. One reversible and two nonreversible mechanisms were identified. K(a) for reversible activation (approximately 100 micro M) depended on cytoplasmic [Ca(2+)] and the bilayer composition. Replacement of neutral lipids by negative phosphatidylserine increased K(a) fourfold, suggesting that reversible binding sites are near the bilayer surface. Suramin and the stilbene derivatives adsorbed to neutral bilayers with maximal mole fractions between 1-8% and with affinities approximately 100 micro M but did not adsorb to negative lipids. DIDS activated RyRs by two nonreversible mechanisms, distinguishable by their disparate DIDS binding rates (10(5) and 60 M(-1) s(-1)) and actions. Both mechanisms activated RyRs via several jumps in open probability, indicating several DIDS binding events. The fast and slow mechanisms are independent of each other, the reversible mechanism and ATP binding. The fast mechanism confers DIDS sensitivity approximately 1000-fold greater than previously reported, increases Ca(2+) activation and increases K(i) for Ca(2+)/Mg(2+) inhibition 10-fold. The slow mechanism activates RyRs in the absence of Ca(2+) and ATP, increases ATP activation without altering K(a), and slightly increases activity at pH < 6.5. These findings explain how different types of DIDS activation are observed under different conditions.
Figures



Similar articles
-
Similarities in the effects of DIDS, DBDS and suramin on cardiac ryanodine receptor function.J Membr Biol. 1999 Mar 15;168(2):159-68. doi: 10.1007/s002329900506. J Membr Biol. 1999. PMID: 10089236
-
Disulfonic stilbene permeation and block of the anion channel from the sarcoplasmic reticulum of rabbit skeletal muscle.Am J Physiol Cell Physiol. 2006 Jun;290(6):C1666-77. doi: 10.1152/ajpcell.00299.2005. Epub 2006 Jan 18. Am J Physiol Cell Physiol. 2006. PMID: 16421208
-
Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters.J Gen Physiol. 2004 Dec;124(6):741-58. doi: 10.1085/jgp.200409092. Epub 2004 Nov 15. J Gen Physiol. 2004. PMID: 15545399 Free PMC article.
-
Short- and long-term functional alterations of the skeletal muscle calcium release channel (Ryanodine receptor) by Suramin: apparent dissociation of single channel current recording and [3H]ryanodine binding.Mol Pharmacol. 2001 Mar;59(3):543-56. doi: 10.1124/mol.59.3.543. Mol Pharmacol. 2001. PMID: 11179450
-
Use-dependent inhibition of the skeletal muscle ryanodine receptor by the suramin analogue NF676.Br J Pharmacol. 2005 Oct;146(4):525-33. doi: 10.1038/sj.bjp.0706359. Br J Pharmacol. 2005. PMID: 16056233 Free PMC article.
Cited by
-
Transient receptor potential vanilloid channel 2 contributes to multi-modal endoplasmic reticulum and perinuclear space dilations that can also be observed in prion-infected mice.Acta Neuropathol. 2025 Jun 23;149(1):68. doi: 10.1007/s00401-025-02906-2. Acta Neuropathol. 2025. PMID: 40549026
-
The modulation of calcium and chloride channels induces cardiomyocytes from human pluripotent stem cells.Int J Biol Sci. 2025 Jan 1;21(1):95-108. doi: 10.7150/ijbs.95568. eCollection 2025. Int J Biol Sci. 2025. PMID: 39744425 Free PMC article.
-
Aldolase potentiates DIDS activation of the ryanodine receptor in rabbit skeletal sarcoplasmic reticulum.Biochem J. 2006 Oct 15;399(2):325-33. doi: 10.1042/BJ20060701. Biochem J. 2006. PMID: 16817780 Free PMC article.
-
Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid.J Membr Biol. 2003 Nov 15;196(2):95-103. doi: 10.1007/s00232-003-0628-9. J Membr Biol. 2003. PMID: 14724746
-
Comparative Analyses of the 12 Most Abundant PCB Congeners Detected in Human Maternal Serum for Activity at the Thyroid Hormone Receptor and Ryanodine Receptor.Environ Sci Technol. 2019 Apr 2;53(7):3948-3958. doi: 10.1021/acs.est.9b00535. Epub 2019 Mar 19. Environ Sci Technol. 2019. PMID: 30821444 Free PMC article.
References
-
- Brooks, S. P., and K. B. Storey. 1992. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 201:119–126. - PubMed
-
- Chu, A., M. C. Dixon, A. Saito, S. Seiler, and S. Fleischer. 1988. Isolation of sarcoplasmic reticulum fractions referable to longitudinal tubules and junctional terminal cisternae from rabbit skeletal muscle. Methods Enzymol. 157:36–50. - PubMed
-
- Coronado, R., J. Morrissette, M. Sukhareva, and D. M. Vaughan. 1994. Structure and function of ryanodine receptors. Am. J. Physiol. 266:C1485–C1504. - PubMed
-
- Grahame, D. C. 1947. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 41:441–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

