Direct activation of gastric H,K-ATPase by N-terminal protein kinase C phosphorylation. Comparison of the acute regulation mechanisms of H,K-ATPase and Na,K-ATPase
- PMID: 12609871
- PMCID: PMC1302738
- DOI: 10.1016/S0006-3495(03)74977-7
Direct activation of gastric H,K-ATPase by N-terminal protein kinase C phosphorylation. Comparison of the acute regulation mechanisms of H,K-ATPase and Na,K-ATPase
Abstract
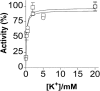
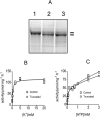
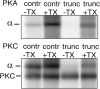
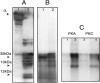
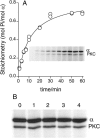
In this study we compared the protein kinase dependent regulation of gastric H,K-ATPase and Na,K-ATPase. The protein kinase A/protein kinase C (PKA/PKC) phosphorylation profile of H,K-ATPase was very similar to the one found in the Na,K-ATPase. PKC phosphorylation was taking place in the N-terminal part of the alpha-subunit with a stoichiometry of approximately 0.6 mol Pi/mole alpha-subunit. PKA phosphorylation was in the C-terminal part and required detergent, as is also found for the Na,K-ATPase. The stoichiometry of PKA-induced phosphorylation was approximately 0.7 mol Pi/mole alpha-subunit. Controlled proteolysis of the N-terminus abolished PKC phosphorylation of native H,K-ATPase. However, after detergent treatment additional C-terminal PKC sites became exposed located at the beginning of the M5M6 hairpin and at the cytoplasmic L89 loop close to the inner face of the plasma membrane. N-terminal PKC phosphorylation of native H,K-ATPase alpha-subunit was found to stimulate the maximal enzyme activity by 40-80% at saturating ATP, depending on pH. Thus, a direct modulation of enzyme activity by PKC phosphorylation could be demonstrated that may be additional to the well-known regulation of acid secretion by recruitment of H,K-ATPase to the apical membranes of the parietal cells. Moreover, a distinct difference in the regulation of H,K-ATPase and Na,K-ATPase is the apparent absence of any small regulatory proteins associated with the H,K-ATPase.
Figures



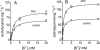
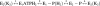

Similar articles
-
Protein kinase C phosphorylation of purified Na,K-ATPase: C-terminal phosphorylation sites at the alpha- and gamma-subunits close to the inner face of the plasma membrane.Biophys J. 2002 Apr;82(4):1907-19. doi: 10.1016/S0006-3495(02)75540-9. Biophys J. 2002. PMID: 11916849 Free PMC article.
-
Functional significance of the shark Na,K-ATPase N-terminal domain. Is the structurally variable N-Terminus involved in tissue-specific regulation by FXYD proteins?Biochemistry. 2005 Oct 4;44(39):13051-62. doi: 10.1021/bi0504456. Biochemistry. 2005. PMID: 16185073
-
alpha 1 but not alpha 2 or alpha 3 isoforms of Na,K-ATPase are efficiently phosphorylated in a novel protein kinase C motif.Biochemistry. 1996 Nov 12;35(45):14098-108. doi: 10.1021/bi960516o. Biochemistry. 1996. PMID: 8916895
-
Phosphorylation of the Na+,K+-ATPase and the H+,K+-ATPase.FEBS Lett. 2010 Jun 18;584(12):2589-95. doi: 10.1016/j.febslet.2010.04.035. Epub 2010 Apr 22. FEBS Lett. 2010. PMID: 20412804 Review.
-
Structural aspects of the gastric H,K ATPase.Ann N Y Acad Sci. 1997 Nov 3;834:65-76. doi: 10.1111/j.1749-6632.1997.tb52226.x. Ann N Y Acad Sci. 1997. PMID: 9405786 Review.
Cited by
-
Key role of phosphorylation sites in ATPase domain and Linker region of MLH1 for DNA binding and functionality of MutLα.Sci Rep. 2023 Aug 2;13(1):12503. doi: 10.1038/s41598-023-39750-x. Sci Rep. 2023. PMID: 37532794 Free PMC article.
-
Adenylyl cyclase 6 is required for maintaining acid-base homeostasis.Clin Sci (Lond). 2018 Aug 30;132(16):1779-1796. doi: 10.1042/CS20180060. Print 2018 Aug 31. Clin Sci (Lond). 2018. PMID: 29941522 Free PMC article.
-
The renal H+-K+-ATPases: physiology, regulation, and structure.Am J Physiol Renal Physiol. 2010 Jan;298(1):F12-21. doi: 10.1152/ajprenal.90723.2008. Epub 2009 Jul 29. Am J Physiol Renal Physiol. 2010. PMID: 19640897 Free PMC article. Review.
-
Evolutionary Analysis of the Lysine-Rich N-terminal Cytoplasmic Domains of the Gastric H+,K+-ATPase and the Na+,K+-ATPase.J Membr Biol. 2018 Dec;251(5-6):653-666. doi: 10.1007/s00232-018-0043-x. Epub 2018 Jul 28. J Membr Biol. 2018. PMID: 30056551
-
Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets.Pharmacol Rev. 2009 Mar;61(1):9-38. doi: 10.1124/pr.108.000711. Pharmacol Rev. 2009. PMID: 19325075 Free PMC article. Review.
References
-
- Asahi, M., Y. Kimura, K. Kurzydlowski, M. Tada, and D. H. MacLennan. 1999. Transmembrane helix M6 in Sarco(endo)plasmic reticulum Ca2+-ATPase forms a functional site with phospholamban. Evidence for a physical interaction at other sites. J. Biol. Chem. 274:32855–32862. - PubMed
-
- Baginski, E. S., P. P. Foa, and B. Zak. 1967. Determination of phosphate: study of labile organic phosphate interference. Clin. Chim. Acta. 14:155–158.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

