The lateral diffusion of selectively aggregated peptides in giant unilamellar vesicles
- PMID: 12609877
- PMCID: PMC1302744
- DOI: 10.1016/s0006-3495(03)74983-2
The lateral diffusion of selectively aggregated peptides in giant unilamellar vesicles
Erratum in
- Biophys J. 2003 Aug;85(2):1338
Abstract
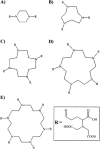
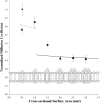
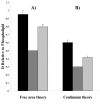
We have systematically investigated the effect of aggregation of a transmembrane peptide on its diffusion in dimyristoylphosphatidylcholine and in palmitoyloleoylphosphatidylcholine model membranes. The hydrophobic segment of the b subunit from E. coli F(1)F(0)-ATP synthase was modified with a histidine tag at the carbonyl terminus and was aggregated selectively by using a series of multivalent, dendritic chelating agents with nitrilotriacetic acid functional groups. Peptide complexes ranging from monomers to hexamers were formed and studied in giant unilamellar vesicles. The rate of diffusion for the transmembrane peptide complexes were found to depend on the size of the complex. The results agree with predictions from the free area model for monomers and dimers, and the hydrodynamic continuum model for tetramers, pentamers, and hexamers. Comparisons with diffusion of lipids confirm that the diffusion of a transmembrane peptide is enhanced by coupling of density fluctuations between the two monolayers.
Figures



Similar articles
-
Nonequilibrium fluctuations of lipid membranes by the rotating motor protein F1F0-ATP synthase.Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):11291-11296. doi: 10.1073/pnas.1701207114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073046 Free PMC article.
-
Lateral diffusion of membrane proteins.J Am Chem Soc. 2009 Sep 9;131(35):12650-6. doi: 10.1021/ja902853g. J Am Chem Soc. 2009. PMID: 19673517
-
Direct interaction of subunits a and b of the F0 complex of Escherichia coli ATP synthase by forming an ab2 subcomplex.J Biol Chem. 2003 Jul 18;278(29):27068-71. doi: 10.1074/jbc.M302027200. Epub 2003 Apr 30. J Biol Chem. 2003. PMID: 12724321
-
Resolving the kinetics of lipid, protein and peptide diffusion in membranes.Mol Membr Biol. 2012 Aug;29(5):118-43. doi: 10.3109/09687688.2012.678018. Epub 2012 May 14. Mol Membr Biol. 2012. PMID: 22582994 Review.
-
Brownian Motion at Lipid Membranes: A Comparison of Hydrodynamic Models Describing and Experiments Quantifying Diffusion within Lipid Bilayers.Biomolecules. 2018 May 22;8(2):30. doi: 10.3390/biom8020030. Biomolecules. 2018. PMID: 29789471 Free PMC article. Review.
Cited by
-
Recent developments in fluorescence correlation spectroscopy for diffusion measurements in planar lipid membranes.Int J Mol Sci. 2010 Jan 28;11(2):427-457. doi: 10.3390/ijms11020427. Int J Mol Sci. 2010. PMID: 20386647 Free PMC article. Review.
-
Lateral membrane diffusion modulated by a minimal actin cortex.Biophys J. 2013 Apr 2;104(7):1465-75. doi: 10.1016/j.bpj.2013.02.042. Biophys J. 2013. PMID: 23561523 Free PMC article.
-
Local mobility in lipid domains of supported bilayers characterized by atomic force microscopy and fluorescence correlation spectroscopy.Biophys J. 2005 Aug;89(2):1081-93. doi: 10.1529/biophysj.105.060327. Epub 2005 May 6. Biophys J. 2005. PMID: 15879469 Free PMC article.
-
N-glycosylation enables high lateral mobility of GPI-anchored proteins at a molecular crowding threshold.Nat Commun. 2016 Sep 19;7:12870. doi: 10.1038/ncomms12870. Nat Commun. 2016. PMID: 27641538 Free PMC article.
-
Inferring diffusion in single live cells at the single-molecule level.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 24;368(1611):20120029. doi: 10.1098/rstb.2012.0029. Print 2013 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23267182 Free PMC article. Review.
References
-
- Adam, G., and M. Delbrück. 1968. Reduction of dimensionality in biological diffusion processes. In Structural Chemistry and Molecular Biology. A. Rich and N. Davidson, editors. W. H. Freeman and Company, San Francisco. 198–215.
-
- Angelova, M. I., and D. S. Dimitrov. 1986. Liposome electroformation. Faraday Discuss. Chem. Soc. 81:303–311.
-
- Angelova, M. I., S. Soléau, Ph. Meléard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC fields: kinetics and application. Prog. Colloid Polym. Sci. 89:127–131.
-
- Axelrod, D. 1985. Fluorescence photobleaching techniques and lateral diffusion. In Spectroscopy and the Dynamics of Molecular Biological Systems. P. Bayley and R. Dale, editors. Academic Press, London. 163–176.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

