Orientation and interactions of an essential tryptophan (Trp-38) in the capsid subunit of Pf3 filamentous virus
- PMID: 12609899
- PMCID: PMC1302766
- DOI: 10.1016/S0006-3495(03)75005-X
Orientation and interactions of an essential tryptophan (Trp-38) in the capsid subunit of Pf3 filamentous virus
Abstract
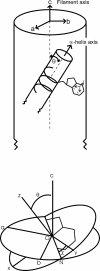
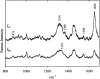
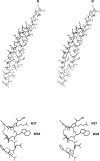
The filamentous bacteriophage Pf3 consists of a covalently closed DNA single strand of 5833 nucleotides sheathed by approximately 2500 copies of a 44-residue capsid subunit. The capsid subunit contains a single tryptophan residue (Trp-38), which is located within the basic C-terminal sequence (-RWIKAQFF) and is essential for virion assembly in vivo. Polarized Raman microspectroscopy has been employed to determine the orientation of the Trp-38 side chain in the native virus structure. The polarized Raman measurements show that the plane of the indolyl ring is tilted by 17 degrees from the virion axis and that the indolyl pseudo-twofold axis is inclined at 46 degrees to the virion axis. Using the presently determined orientation of the indolyl ring and side-chain torsion angles, chi(1) (N-C(alpha)-C(beta)-C(gamma)) and chi(2,1) (C(alpha)-C(beta)-C(gamma)-C(delta1)), we propose a detailed molecular model for the local structure of Trp-38 in the Pf3 virion. The present Pf3 model is consistent with previously reported Raman, ultraviolet-resonance Raman and fluorescence results suggesting an unusual environment for Trp-38 in the virion assembly, probably involving an intrasubunit cation-pi interaction between the guanidinium moiety of Arg-37 and the indolyl moiety of Trp-38. Such a C-terminal Trp-38/Arg-37 interaction may be important for the stabilization of a subunit conformation that is required for binding to the single-stranded DNA genome during virion assembly.
Figures


Similar articles
-
Ultraviolet-resonance raman spectroscopy of the filamentous virus Pf3: interactions of Trp 38 specific to the assembled virion subunit.Biochemistry. 2000 Jan 11;39(1):146-52. doi: 10.1021/bi992018w. Biochemistry. 2000. PMID: 10625489
-
Structural details of the thermophilic filamentous bacteriophage PH75 determined by polarized Raman microspectroscopy.Biochemistry. 2005 Mar 29;44(12):4861-9. doi: 10.1021/bi0479306. Biochemistry. 2005. PMID: 15779912
-
Structure and organization of bacteriophage Pf3 probed by Raman and ultraviolet resonance Raman spectroscopy.Biochemistry. 2001 Jan 16;40(2):449-58. doi: 10.1021/bi0018887. Biochemistry. 2001. PMID: 11148039
-
Orientation of tryptophan-26 in coat protein subunits of the filamentous virus Ff by polarized Raman microspectroscopy.Biochemistry. 1996 Aug 13;35(32):10403-10. doi: 10.1021/bi9527707. Biochemistry. 1996. PMID: 8756696
-
Analysis of X-ray diffraction from fibres of Pf1 Inovirus (filamentous bacteriophage) shows that the DNA in the virion is not highly ordered.J Mol Biol. 1998 Dec 18;284(5):1265-71. doi: 10.1006/jmbi.1998.2275. J Mol Biol. 1998. PMID: 9878347 Review.
Cited by
-
Raman tensors and their application in structural studies of biological systems.Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(3):83-97. doi: 10.2183/pjab.85.83. Proc Jpn Acad Ser B Phys Biol Sci. 2009. PMID: 19282645 Free PMC article. Review.
-
The McdAB system positions α-carboxysomes in proteobacteria.Mol Microbiol. 2021 Jul;116(1):277-297. doi: 10.1111/mmi.14708. Epub 2021 Mar 8. Mol Microbiol. 2021. PMID: 33638215 Free PMC article.
-
Origin and Evolution of Carboxysome Positioning Systems in Cyanobacteria.Mol Biol Evol. 2020 May 1;37(5):1434-1451. doi: 10.1093/molbev/msz308. Mol Biol Evol. 2020. PMID: 31899489 Free PMC article.
-
An invariant C-terminal tryptophan in McdB mediates its interaction and positioning function with carboxysomes.Mol Biol Cell. 2024 Aug 1;35(8):ar107. doi: 10.1091/mbc.E23-11-0443. Epub 2024 Jun 26. Mol Biol Cell. 2024. PMID: 38922842 Free PMC article.
-
Positioning the Model Bacterial Organelle, the Carboxysome.mBio. 2021 May 11;12(3):e02519-19. doi: 10.1128/mBio.02519-19. mBio. 2021. PMID: 33975941 Free PMC article. Review.
References
-
- Arp, Z., D. Autrey, J. Laane, S. A. Overman, and G. J. Thomas, Jr. 2001. Tyrosine Raman signatures of the filamentous virus Ff are diagnostic of non-hydrogen-bonded phenoxyls: demonstration by Raman and infrared spectroscopy of p-Cresol vapor. Biochemistry. 40:2522–2529. - PubMed
-
- Benevides, J. M., M. Tsuboi, A. H. J. Wang, and G. J. Thomas, Jr. 1993. Local Raman tensors of double-helical DNA in the crystal: a basis for determining DNA residue orientations. J. Am. Chem. Soc. 115:5351–5359.
-
- Blanch, E. W., A. F. Bell, L. Hecht, L. A. Day, and L. D. Barron. 1999. Raman optical activity of filamentous bacteriophages: hydration of alpha-helices. J. Mol. Biol. 290:1–7. - PubMed
-
- Blanch, E. W., L. Hecht, L. A. Day, D. M. Pederson, and L. D. Barron. 2001. Tryptophan absolute stereochemistry in viral coat proteins from Raman optical activity. J. Am. Chem. Soc. 123:4863–4864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

