Ionic mechanisms underlying spontaneous CA1 neuronal firing in Ca2+-free solution
- PMID: 12609911
- PMCID: PMC1302778
- DOI: 10.1016/S0006-3495(03)75017-6
Ionic mechanisms underlying spontaneous CA1 neuronal firing in Ca2+-free solution
Abstract
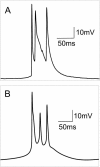
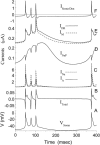
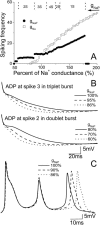
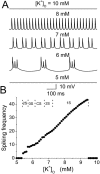
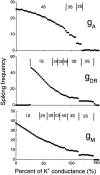
Hippocampal CA1 neurons exposed to zero-[Ca(2+)] solutions can generate periodic spontaneous synchronized activity in the absence of synaptic function. Experiments using hippocampal slices showed that, after exposure to zero-[Ca(2+)](0) solution, CA1 pyramidal cells depolarized 5-10 mV and started firing spontaneous action potentials. Spontaneous single neuron activity appeared in singlets or was grouped into bursts of two or three action potentials. A 16-compartment, 23-variable cable model of a CA1 pyramidal neuron was developed to study mechanisms of spontaneous neuronal bursting in a calcium-free extracellular solution. In the model, five active currents (a fast sodium current, a persistent sodium current, an A-type transient potassium current, a delayed rectifier potassium current, and a muscarinic potassium current) are included in the somatic compartment. The model simulates the spontaneous bursting behavior of neurons in calcium-free solutions. The mechanisms underlying several aspects of bursting are studied, including the generation of triplet bursts, spike duration, burst termination, after-depolarization behavior, and the prolonged inactive period between bursts. We show that the small persistent sodium current can play a key role in spontaneous CA1 activity in zero-calcium solutions. In particular, it is necessary for the generation of an after-depolarizing potential and prolongs both individual bursts and the interburst interval.
Figures
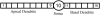



Similar articles
-
Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study.J Neurophysiol. 2006 Oct;96(4):1912-26. doi: 10.1152/jn.00205.2006. Epub 2006 Jun 28. J Neurophysiol. 2006. PMID: 16807352
-
Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells.J Physiol. 2005 Aug 1;566(Pt 3):689-715. doi: 10.1113/jphysiol.2005.086835. Epub 2005 May 12. J Physiol. 2005. PMID: 15890705 Free PMC article.
-
A unified model of CA1/3 pyramidal cells: an investigation into excitability.Prog Biophys Mol Biol. 2011 Mar;105(1-2):34-48. doi: 10.1016/j.pbiomolbio.2010.09.020. Epub 2010 Sep 29. Prog Biophys Mol Biol. 2011. PMID: 20887748
-
Imaging Native Calcium Currents in Brain Slices.Adv Exp Med Biol. 2020;1131:73-91. doi: 10.1007/978-3-030-12457-1_4. Adv Exp Med Biol. 2020. PMID: 31646507 Review.
-
Spontaneous signalling in small central neurons: mechanisms and roles of spike-amplitude and spike-interval fluctuations.Int J Neural Syst. 1996 Sep;7(4):369-76. doi: 10.1142/s0129065796000336. Int J Neural Syst. 1996. PMID: 8968826 Review.
Cited by
-
An ephaptic transmission model of CA3 pyramidal cells: an investigation into electric field effects.Cogn Neurodyn. 2014 Jun;8(3):177-97. doi: 10.1007/s11571-013-9269-6. Epub 2013 Oct 5. Cogn Neurodyn. 2014. PMID: 24808928 Free PMC article.
-
Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission.J Neurosci. 2014 Jan 22;34(4):1409-19. doi: 10.1523/JNEUROSCI.3877-13.2014. J Neurosci. 2014. PMID: 24453330 Free PMC article.
-
Diffusive coupling and network periodicity: a computational study.Biophys J. 2008 Aug;95(3):1126-37. doi: 10.1529/biophysj.108.129239. Epub 2008 Apr 25. Biophys J. 2008. PMID: 18441034 Free PMC article.
-
The role of coupling strength and internal delay between compartments in shaping the bursting behavior of cortical neuron.Neurol Sci. 2014 Jun;35(6):883-9. doi: 10.1007/s10072-013-1619-y. Epub 2014 Jan 9. Neurol Sci. 2014. PMID: 24402782
-
Enhanced multiple vibrational resonances by Na+ and K+ dynamics in a neuron model.Sci Rep. 2015 Jan 8;5:7684. doi: 10.1038/srep07684. Sci Rep. 2015. PMID: 25567752 Free PMC article.
References
-
- Aghajanian, G. K., and K. Rasmussen. 1989. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult-rat brain-slices. Synapse. 3:331–338. - PubMed
-
- Bernard, C., R. C. Cannon, Y. B. Ari, and H. V. Wheal. 1997. Model of septio-temporal propagation of action potentials in the Schaffer collateral pathway of the CA1 area of the rat hippocampus. Hippocampus. 7:58–72. - PubMed
-
- Bikson, M., R. S. Ghai, S. C. Baraban, and D. M. Durand. 1999. Modulation of burst frequency, duration, and amplitude in the zero-Ca2+ model of epileptiform activity. J. Neurophys. 82:2262–2270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

