Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses
- PMID: 12609941
- PMCID: PMC150178
- DOI: 10.1136/bmj.326.7387.472
Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses
Abstract
Objective: To determine the validity of adjusted indirect comparisons by using data from published meta-analyses of randomised trials.
Design: Direct comparison of different interventions in randomised trials and adjusted indirect comparison in which two interventions were compared through their relative effect versus a common comparator. The discrepancy between the direct and adjusted indirect comparison was measured by the difference between the two estimates.
Data sources: Database of abstracts of reviews of effectiveness (1994-8), the Cochrane database of systematic reviews, Medline, and references of retrieved articles.
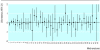
Results: 44 published meta-analyses (from 28 systematic reviews) provided sufficient data. In most cases, results of adjusted indirect comparisons were not significantly different from those of direct comparisons. A significant discrepancy (P<0.05) was observed in three of the 44 comparisons between the direct and the adjusted indirect estimates. There was a moderate agreement between the statistical conclusions from the direct and adjusted indirect comparisons (kappa 0.51). The direction of discrepancy between the two estimates was inconsistent.
Conclusions: Adjusted indirect comparisons usually but not always agree with the results of head to head randomised trials. When there is no or insufficient direct evidence from randomised trials, the adjusted indirect comparison may provide useful or supplementary information on the relative efficacy of competing interventions. The validity of the adjusted indirect comparisons depends on the internal validity and similarity of the included trials.
Figures


References
-
- Pocock SJ. Clinical trials: a practical approach. New York: John Wiley; 1996.
-
- Hasselblad V, Kong DF. Statistical methods for comparison to placebo in active-control trials. Drug Inf J. 2001;35:435–449.
-
- Fisher LD, Gent M, Buller HR. Active-control trials: how would a new agent compare with placebo? A method illustrated with clopidogrel, aspirin, and placebo. Am Heart J. 2001;141:26–32. - PubMed
-
- McAlister F, Laupacis A, Wells G, Sackett D. Users' guides to the medical literature: XIX. Applying clinical trial results B. Guidelines for determining whether a drug is exerting (more than) a class effect. JAMA. 1999;282:1371–1377. - PubMed
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
