The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling
- PMID: 12610111
- PMCID: PMC149518
- DOI: 10.1128/jvi.77.6.3360-3370.2003
The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling
Abstract
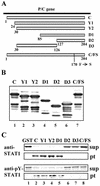
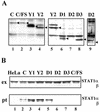
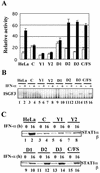
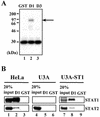
Sendai virus (SeV) C protein functions as an interferon (IFN) antagonist and renders cells unresponsive to both alpha/beta IFN (IFN-alpha/beta) and IFN-gamma. We have recently found the physical association of the C protein with signal transducer and activator of transcription 1 (STAT1) in infected cells. However, involvement of the C-STAT1 interaction in the blockade of IFN signaling has remained unclear. We generated here a series of C mutant proteins that retained or lost the STAT1-binding capacity and examined their effects on IFN-alpha signaling. All of the C mutant proteins with no STAT1-binding capacity lost the ability to inhibit the IFN-alpha response. In contrast, the C mutant proteins retaining the STAT1-binding capacity suppressed IFN-alpha-stimulated tyrosine phosphorylation of both STAT2 and STAT1 to various degrees. Remarkably, their anti-IFN-alpha capacities correlated well with the inhibitory effect on phosphorylation of STAT2 rather than STAT1. In infected cells, the levels of tyrosine-phosphorylated (pY) STAT2 were below the detection level irrespective of duration of IFN-alpha stimulation, whereas the levels of pY-STAT1 strikingly increased after long-term IFN-alpha stimulation. These results suggest that the STAT2 activation process is a crucial target for the blockade of IFN-alpha signaling. An in vitro binding assay with extracts from (STAT1-deficient) U3A and (STAT1-expressing) U3A-ST1 cells suggested the requirement of STAT1 for the C-STAT2 interaction. Furthermore, expression of STAT1 enhanced the inhibitory effect of the C protein on STAT2 activation in U3A cells. The C protein thus appears to participate in the inhibitory process for STAT2 activation through the STAT1 interaction.
Figures




Similar articles
-
Structural Basis of the Inhibition of STAT1 Activity by Sendai Virus C Protein.J Virol. 2015 Nov;89(22):11487-99. doi: 10.1128/JVI.01887-15. Epub 2015 Sep 2. J Virol. 2015. PMID: 26339056 Free PMC article.
-
Interferon-gamma inhibits interferon-alpha signalling in hepatic cells: evidence for the involvement of STAT1 induction and hyperexpression of STAT1 in chronic hepatitis C.Biochem J. 2004 Apr 1;379(Pt 1):199-208. doi: 10.1042/BJ20031495. Biochem J. 2004. PMID: 14690454 Free PMC article.
-
Characterization of the amino acid residues of sendai virus C protein that are critically involved in its interferon antagonism and RNA synthesis down-regulation.J Virol. 2004 Jul;78(14):7443-54. doi: 10.1128/JVI.78.14.7443-7454.2004. J Virol. 2004. PMID: 15220418 Free PMC article.
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
-
STATs find that hanging together can be stimulating.Science. 1996 Aug 9;273(5276):750-1. doi: 10.1126/science.273.5276.750. Science. 1996. PMID: 8701326 Review. No abstract available.
Cited by
-
Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins.Front Microbiol. 2022 Jan 31;12:790191. doi: 10.3389/fmicb.2021.790191. eCollection 2021. Front Microbiol. 2022. PMID: 35173691 Free PMC article. Review.
-
Human parainfluenza virus type 4 is incapable of evading the interferon-induced antiviral effect.J Virol. 2005 Dec;79(23):14756-68. doi: 10.1128/JVI.79.23.14756-14768.2005. J Virol. 2005. PMID: 16282476 Free PMC article.
-
A Single Amino Acid Substitution within the Paramyxovirus Sendai Virus Nucleoprotein Is a Critical Determinant for Production of Interferon-Beta-Inducing Copyback-Type Defective Interfering Genomes.J Virol. 2018 Feb 12;92(5):e02094-17. doi: 10.1128/JVI.02094-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237838 Free PMC article.
-
Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function.J Virol. 2004 Nov;78(21):11632-40. doi: 10.1128/JVI.78.21.11632-11640.2004. J Virol. 2004. PMID: 15479804 Free PMC article.
-
Inhibition of interferon-mediated antiviral activity by murine gammaherpesvirus 68 latency-associated M2 protein.J Virol. 2004 Nov;78(22):12416-27. doi: 10.1128/JVI.78.22.12416-12427.2004. J Virol. 2004. PMID: 15507628 Free PMC article.
References
-
- Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. - PMC - PubMed
-
- Asao, H., and X. Y. Fu. 2000. Interferon-gamma has dual potentials in inhibiting or promoting cell proliferation. J. Biol. Chem. 275:867-874. - PubMed
-
- Chatziandreou, N., D. Young, J. Andrejeva, S. Goodbourn, and R. E. Randall. 2002. Differences in interferon sensitivity and biological properties of two related isolates of simian virus 5: a model for virus persistence. Virology 293:234-242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

