The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells
- PMID: 12610127
- PMCID: PMC149500
- DOI: 10.1128/jvi.77.6.3516-3530.2003
The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells
Abstract
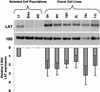
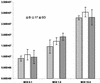
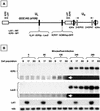
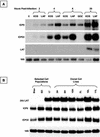
During latency, herpes simplex virus expresses a unique set of latency-associated transcripts (LATs). As the 2.0-kb LAT intron is complementary to, and overlaps, the 3' end of the ICP0 transcript, it has been suggested that the stable LAT intron might function as an antisense repressor of ICP0 expression. We tested this hypothesis in cell culture by dissociating cis- and trans-acting effects of the 2.0-kb LAT, using a series of complementary strategies. Initially, we constructed 293T cell lines that stably express the nuclear 2.0-kb LAT intron to determine whether LAT accumulation in trans affects ICP0 expression. ICP0 mRNA and protein expression profiles were studied (i) following infections with a viral mutant containing wild-type LAT and ICP0 sequences but having deletions of other immediate-early (IE) genes, thus preventing the progression of viral early gene expression, (ii) at early time points after infection with wild-type virus, before viral LAT expression, and (iii) by plasmid transfections. Northern and Western blot analysis showed that trans expression of the 2.0-kb LAT intron does not affect ICP0 mRNA expression, stability, accumulation, splicing, or translation. In addition, suppression of viral replication by overexpression of the 2.0-kb LAT, which has been detected previously in neuronal cell lines, was not found in these nonneuronal cell lines. However, deletion of the latency-active promoter (LAP) region of the virus resulted in overexpression of IE genes, which occurred soon after infection, before viral LAT expression had commenced. This was not complemented by the expression of LAT in trans, suggesting that the LAP deletion affected transcriptional regulation of the IE genes in cis. We conclude that the function of the highly conserved LAT intron is unlikely to involve a direct-acting anti-ICP0 antisense mechanism but that the LAT region could affect ICP0 mRNA expression from the viral genome.
Figures






References
-
- Block, T. M., S. Deshmane, J. Masonis, J. Maggioncalda, T. Valyi Nagi, and N. W. Fraser. 1993. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology 192:618-630. - PubMed
-
- Burton, E. A., S. Huang, W. F. Goins, and J. C. Glorioso. 2003. Use of the herpes simplex viral genome to construct gene therapy vectors. Methods Mol. Med. 76:1-31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

