The 5'-end sequence of the genome of Aichi virus, a picornavirus, contains an element critical for viral RNA encapsidation
- PMID: 12610129
- PMCID: PMC149490
- DOI: 10.1128/jvi.77.6.3542-3548.2003
The 5'-end sequence of the genome of Aichi virus, a picornavirus, contains an element critical for viral RNA encapsidation
Abstract
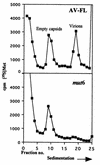
Picornavirus positive-strand RNAs are selectively encapsidated despite the coexistence of viral negative-strand RNAs and cellular RNAs in infected cells. However, the precise mechanism of the RNA encapsidation process in picornaviruses remains unclear. Here we report the first identification of an RNA element critical for encapsidation in picornaviruses. The 5' end of the genome of Aichi virus, a member of the family Picornaviridae, folds into three stem-loop structures (SL-A, SL-B, and SL-C, from the most 5' end). In the previous study, we constructed a mutant, termed mut6, by exchanging the seven-nucleotide stretches of the middle part of the stem in SL-A with each other to maintain the base pairings of the stem. mut6 exhibited efficient RNA replication and translation but formed no plaques. The present study showed that in cells transfected with mut6 RNA, empty capsids were accumulated, but few virions containing RNA were formed. This means that mut6 has a severe defect in RNA encapsidation. Site-directed mutational analysis indicated that as the mutated region was narrowed, the encapsidation was improved. As a result, the mutation of the 7 bp of the middle part of the stem in SL-A was required for abolishing the plaque-forming ability. Thus, the 5'-end sequence of the Aichi virus genome was shown to play an important role in encapsidation.
Figures



Similar articles
-
The 5'-terminal region of the Aichi virus genome encodes cis-acting replication elements required for positive- and negative-strand RNA synthesis.J Virol. 2005 Jun;79(11):6918-31. doi: 10.1128/JVI.79.11.6918-6931.2005. J Virol. 2005. PMID: 15890931 Free PMC article.
-
Functional analysis of the stem-loop structures at the 5' end of the Aichi virus genome.Virology. 2003 Aug 15;313(1):56-65. doi: 10.1016/s0042-6822(03)00346-5. Virology. 2003. PMID: 12951021
-
Construction of an infectious cDNA clone of Aichi virus (a new member of the family Picornaviridae) and mutational analysis of a stem-loop structure at the 5' end of the genome.J Virol. 2001 Sep;75(17):8021-30. doi: 10.1128/jvi.75.17.8021-8030.2001. J Virol. 2001. PMID: 11483747 Free PMC article.
-
Picornavirus IRES: structure function relationship.Curr Pharm Des. 2004;10(30):3757-67. doi: 10.2174/1381612043382657. Curr Pharm Des. 2004. PMID: 15579069 Review.
-
Cis-active RNA elements (CREs) and picornavirus RNA replication.Virus Res. 2009 Feb;139(2):240-52. doi: 10.1016/j.virusres.2008.07.027. Epub 2008 Sep 20. Virus Res. 2009. PMID: 18773930 Free PMC article. Review.
Cited by
-
ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites.EMBO J. 2012 Feb 1;31(3):754-66. doi: 10.1038/emboj.2011.429. Epub 2011 Nov 29. EMBO J. 2012. PMID: 22124328 Free PMC article.
-
Aichi virus leader protein is involved in viral RNA replication and encapsidation.J Virol. 2003 Oct;77(20):10799-807. doi: 10.1128/jvi.77.20.10799-10807.2003. J Virol. 2003. PMID: 14512530 Free PMC article.
-
The long-lasting enigma of polycytidine (polyC) tract.PLoS Pathog. 2021 Aug 4;17(8):e1009739. doi: 10.1371/journal.ppat.1009739. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34347852 Free PMC article. Review.
-
A hepatitis C virus cis-acting replication element forms a long-range RNA-RNA interaction with upstream RNA sequences in NS5B.J Virol. 2008 Sep;82(18):9008-22. doi: 10.1128/JVI.02326-07. Epub 2008 Jul 9. J Virol. 2008. PMID: 18614633 Free PMC article.
-
Structure of Ljungan virus provides insight into genome packaging of this picornavirus.Nat Commun. 2015 Oct 8;6:8316. doi: 10.1038/ncomms9316. Nat Commun. 2015. PMID: 26446437 Free PMC article.
References
-
- Andino, R., G. E. Rieckhof, and D. Baltimore. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63:369-380. - PubMed
-
- Ansardi, D. C., Z. Moldoveane, D. C. Porter, D. E. Walker, R. M. Conry, A. F. LoBuglio, S. McPherson, and C. D. Morrow. 1994. Characterization of poliovirus replicons encoding carcinoembryonic antigen. Cancer Res. 54:6359-6364. - PubMed
-
- Barclay, W., Q. Li, G. Hutchinson, D. Moon, A. Richardson, N. Percy, J. W. Almond, and D. J. Evans. 1998. Encapsidation studies of poliovirus subgenomic replicons. J. Gen. Virol. 79:1725-1734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

