Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation
- PMID: 12610139
- PMCID: PMC149489
- DOI: 10.1128/jvi.77.6.3634-3646.2003
Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation
Abstract
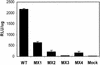
Two highly conserved cationic amphipathic alpha-helical motifs, designated lentivirus lytic peptides 1 and 2 (LLP-1 and LLP-2), have been characterized in the carboxyl terminus of the transmembrane (TM) envelope glycoprotein (Env) of lentiviruses. Although various properties have been attributed to these domains, their structural and functional significance is not clearly understood. To determine the specific contributions of the Env LLP domains to Env expression, processing, and incorporation and to viral replication and syncytium induction, site-directed LLP mutants of a primary dualtropic infectious human immunodeficiency virus type 1 (HIV-1) isolate (ME46) were examined. Substitutions were made for highly conserved arginine residues in either the LLP-1 or LLP-2 domain (MX1 or MX2, respectively) or in both domains (MX4). The HIV-1 mutants with altered LLP domains demonstrated distinct phenotypes. The LLP-1 mutants (MX1 and MX4) were replication defective and showed an average of 85% decrease in infectivity, which was associated with an evident decrease in gp41 incorporation into virions without a significant decrease in Env expression or processing in transfected 293T cells. In contrast, MX2 virus was replication competent and incorporated a full complement of Env into its virions, indicating a differential role for the LLP-1 domain in Env incorporation. Interestingly, the replication-competent MX2 virus was impaired in its ability to induce syncytia in T-cell lines. This defect in cell-cell fusion did not correlate with apparent defects in the levels of cell surface Env expression, oligomerization, or conformation. The lack of syncytium formation, however, correlated with a decrease of about 90% in MX2 Env fusogenicity compared to that of wild-type Env in quantitative luciferase-based cell-cell fusion assays. The LLP-1 mutant MX1 and MX4 Envs also exhibited an average of 80% decrease in fusogenicity. Altogether, these results demonstrate for the first time that the highly conserved LLP domains perform critical but distinct functions in Env incorporation and fusogenicity.
Figures







Similar articles
-
Differential functional phenotypes of two primary HIV-1 strains resulting from homologous point mutations in the LLP domains of the envelope gp41 intracytoplasmic domain.Virology. 2007 Oct 10;367(1):102-16. doi: 10.1016/j.virol.2007.05.027. Epub 2007 Jun 19. Virology. 2007. PMID: 17582453 Free PMC article.
-
Unique functional properties of conserved arginine residues in the lentivirus lytic peptide domains of the C-terminal tail of HIV-1 gp41.J Biol Chem. 2014 Mar 14;289(11):7630-40. doi: 10.1074/jbc.M113.529339. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497632 Free PMC article.
-
Effect of point mutations in the N terminus of the lentivirus lytic peptide-1 sequence of human immunodeficiency virus type 1 transmembrane protein gp41 on Env stability.J Biol Chem. 2002 May 3;277(18):15363-75. doi: 10.1074/jbc.M201479200. Epub 2002 Feb 21. J Biol Chem. 2002. PMID: 11859090
-
Cytopathic mechanisms of HIV-1.Virol J. 2007 Oct 18;4:100. doi: 10.1186/1743-422X-4-100. Virol J. 2007. PMID: 17945027 Free PMC article. Review.
-
HIV-1 replication.Somat Cell Mol Genet. 2001 Nov;26(1-6):13-33. doi: 10.1023/a:1021070512287. Somat Cell Mol Genet. 2001. PMID: 12465460 Review.
Cited by
-
HIV-1 Envelope Glycoprotein Amino Acids Signatures Associated with Clade B Transmitted/Founder and Recent Viruses.Viruses. 2019 Nov 1;11(11):1012. doi: 10.3390/v11111012. Viruses. 2019. PMID: 31683782 Free PMC article.
-
Effects of stabilization of the gp41 cytoplasmic domain on fusion activity and infectivity of SIVmac239.AIDS Res Hum Retroviruses. 2011 Nov;27(11):1213-22. doi: 10.1089/AID.2010.0321. Epub 2011 May 4. AIDS Res Hum Retroviruses. 2011. PMID: 21434848 Free PMC article.
-
HIV control is mediated in part by CD8+ T-cell targeting of specific epitopes.J Virol. 2014 Nov;88(22):12937-48. doi: 10.1128/JVI.01004-14. Epub 2014 Aug 27. J Virol. 2014. PMID: 25165115 Free PMC article.
-
C-terminal tail of human immunodeficiency virus gp41: functionally rich and structurally enigmatic.J Gen Virol. 2013 Jan;94(Pt 1):1-19. doi: 10.1099/vir.0.046508-0. Epub 2012 Oct 17. J Gen Virol. 2013. PMID: 23079381 Free PMC article. Review.
-
Membrane structure correlates to function of LLP2 on the cytoplasmic tail of HIV-1 gp41 protein.Biophys J. 2013 Aug 6;105(3):657-66. doi: 10.1016/j.bpj.2013.06.042. Biophys J. 2013. PMID: 23931314 Free PMC article.
References
-
- Beary, T. P., S. B. Tencza, T. A. Mietzner, and R. C. Montelaro. 1998. Interruption of T-cell signal transduction by lentivirus lytic peptides from HIV-1 transmembrane protein. J. Pept. Res. 51:75-79. - PubMed
-
- Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. - PMC - PubMed
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
-
- Bosch, M. L., P. L. Earl, K. Fargnoli, S. Picciafuoco, F. Giombini, F. Wong-Staal, and G. Franchini. 1989. Identification of the fusion peptide of primate immunodeficiency viruses. Science 244:694-697. - PubMed
-
- Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, and A. Jungbauer. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

