The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion
- PMID: 12612079
- PMCID: PMC149462
- DOI: 10.1128/MCB.23.6.2068-2082.2003
The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion
Abstract
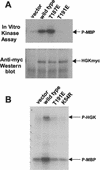
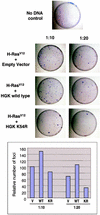
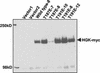
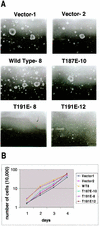
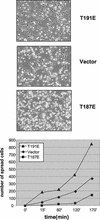
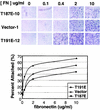
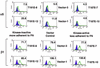
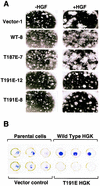
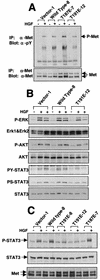
HGK (hepatocyte progenitor kinase-like/germinal center kinase-like kinase) is a member of the human STE20/mitogen-activated protein kinase kinase kinase kinase family of serine/threonine kinases and is the ortholog of mouse NIK (Nck-interacting kinase). We have cloned a novel splice variant of HGK from a human tumor line and have further identified a complex family of HGK splice variants. We showed HGK to be highly expressed in most tumor cell lines relative to normal tissue. An active role for this kinase in transformation was suggested by an inhibition of H-Ras(V12)-induced focus formation by expression of inactive, dominant-negative mutants of HGK in both fibroblast and epithelial cell lines. Expression of an inactive mutant of HGK also inhibited the anchorage-independent growth of cells yet had no effect on proliferation in monolayer culture. Expression of HGK mutants modulated integrin receptor expression and had a striking effect on hepatocyte growth factor-stimulated epithelial cell invasion. Together, these results suggest an important role for HGK in cell transformation and invasiveness.
Figures


References
-
- Bagrodia, S., and R. A. Cerione. 1999. Pak to the future. Trends Cell Biol. 9:350-355. - PubMed
-
- Bao, Q., and R. C. Hughes. 1995. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J. Cell Sci. 108:2791-2800. - PubMed
-
- Berman, A. E., and N. I. Kozlova. 2000. Integrins: structure and functions. Membrane Cell Biol. 13:207-244. - PubMed
-
- Bischoff, J. R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, C. S. Chan, M. Novotny, D. J. Slamon, and G. D. Plowman. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17:3052-3065. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
