Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding
- PMID: 12612087
- PMCID: PMC149465
- DOI: 10.1128/MCB.23.6.2171-2181.2003
Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding
Abstract
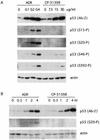
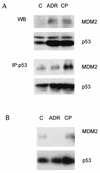
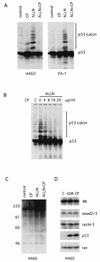
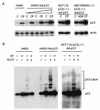
CP-31398, a styrylquinazoline, emerged from a high throughput screen for therapeutic agents that restore a wild-type-associated epitope (monoclonal antibody 1620) on the DNA-binding domain of the p53 protein. We found that CP-31398 can not only restore p53 function in mutant p53-expressing cells but also significantly increase the protein level and promote the activity of wild-type p53 in multiple human cell lines, including ATM-null cells. Cells treated with CP-31398 undergo either cell cycle arrest or apoptosis. Further investigation showed that CP-31398 blocks the ubiquitination and degradation of p53 but not in human papillomavirus E6-expressing cells. Of note, CP-31398 does not block the physical association between p53 and MDM2 in vivo. Moreover, unlike the DNA-damaging agent adriamycin, which induces strong phosphorylation of p53 on serines 15 and 20, CP-31398 exposure leads to no measurable phosphorylation on these sites. We found that CP-31398 could also stabilize exogenous p53 in p53 mutant, wild-type, and p53-null human cells, even in MDM2-null p53(-/-) mouse embryonic fibroblasts. Our results suggest a model wherein CP-31398-mediated stabilization of p53 may result from reduced ubiquitination, leading to high levels of transcriptionally active p53. Further understanding of this mechanism may lead to novel strategies for p53 stabilization and tumor suppression in cancers, even those with absent ARF or high MDM2 expression.
Figures



Similar articles
-
The mutant p53-conformation modifying drug, CP-31398, can induce apoptosis of human cancer cells and can stabilize wild-type p53 protein.Cancer Biol Ther. 2002 Jan-Feb;1(1):47-55. doi: 10.4161/cbt.1.1.41. Cancer Biol Ther. 2002. PMID: 12174820
-
Limited role of N-terminal phosphoserine residues in the activation of transcription by p53.Oncogene. 2004 May 27;23(25):4477-87. doi: 10.1038/sj.onc.1207575. Oncogene. 2004. PMID: 15064747
-
p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3.Oncogene. 2002 May 2;21(19):3082-8. doi: 10.1038/sj.onc.1205426. Oncogene. 2002. PMID: 12082540
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
How to activate p53.Curr Biol. 2000 Apr 20;10(8):R315-7. doi: 10.1016/s0960-9822(00)00439-5. Curr Biol. 2000. PMID: 10801407 Review.
Cited by
-
Folding and misfolding mechanisms of the p53 DNA binding domain at physiological temperature.Protein Sci. 2006 Nov;15(11):2457-65. doi: 10.1110/ps.062324206. Epub 2006 Sep 25. Protein Sci. 2006. PMID: 17001034 Free PMC article.
-
Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics.Adv Pharm Bull. 2019 Jun;9(2):205-218. doi: 10.15171/apb.2019.024. Epub 2019 Jun 1. Adv Pharm Bull. 2019. PMID: 31380246 Free PMC article. Review.
-
Mutant p53 targeting by the low molecular weight compound STIMA-1.Mol Oncol. 2008 Jun;2(1):70-80. doi: 10.1016/j.molonc.2008.02.004. Epub 2008 Mar 7. Mol Oncol. 2008. PMID: 19383329 Free PMC article.
-
A p53-stabilizing agent, CP-31398, induces p21 expression with increased G2/M phase through the YY1 transcription factor in esophageal carcinoma defective of the p53 pathway.Am J Cancer Res. 2019 Jan 1;9(1):79-93. eCollection 2019. Am J Cancer Res. 2019. PMID: 30755813 Free PMC article.
-
Regulation of protein quality control by UBE4B and LSD1 through p53-mediated transcription.PLoS Biol. 2015 Apr 2;13(4):e1002114. doi: 10.1371/journal.pbio.1002114. eCollection 2015 Apr. PLoS Biol. 2015. PMID: 25837623 Free PMC article.
References
-
- An, W. G., Y. Chuman, T. Fojo, and M. V. Blagosklonny. 1998. Inhibitors of transcription, proteasome inhibitors, and DNA-damaging drugs differentially affect feedback of p53 degradation. Exp. Cell Res. 244:54-60. - PubMed
-
- Banerjee, D., and A. Liefshitz. 2001. Potential of the proteasomal inhibitor MG-132 as an anticancer agent, alone and in combination. Anticancer Res. 21:3941-3947. - PubMed
-
- Bean, L. J., and G. R. Stark. 2001. Phosphorylation of serines 15 and 37 is necessary for efficient accumulation of p53 following irradiation with UV. Oncogene 20:1076-1084. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
