Defective DNA repair and increased genomic instability in Artemis-deficient murine cells
- PMID: 12615897
- PMCID: PMC2193825
- DOI: 10.1084/jem.20021891
Defective DNA repair and increased genomic instability in Artemis-deficient murine cells
Abstract
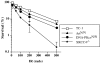
In developing lymphocytes, the recombination activating gene endonuclease cleaves DNA between V, D, or J coding and recombination signal (RS) sequences to form hairpin coding and blunt RS ends, which are fused to form coding and RS joins. Nonhomologous end joining (NHEJ) factors repair DNA double strand breaks including those induced during VDJ recombination. Human radiosensitive severe combined immunodeficiency results from lack of Artemis function, an NHEJ factor with in vitro endonuclease/exonuclease activities. We inactivated Artemis in murine embryonic stem (ES) cells by targeted mutation. Artemis deficiency results in impaired VDJ coding, but not RS, end joining. In addition, Artemis-deficient ES cells are sensitive to a radiomimetic drug, but less sensitive to ionizing radiation. VDJ coding joins from Artemis-deficient ES cells, which surprisingly are distinct from the highly deleted joins consistently obtained from DNA-dependent protein kinase catalytic subunit-deficient ES cells, frequently lack deletions and often display large junctional palindromes, consistent with a hairpin coding end opening defect. Strikingly, Artemis-deficient ES cells have increased chromosomal instability including telomeric fusions. Thus, Artemis appears to be required for a subset of NHEJ reactions that require end processing. Moreover, Artemis functions as a genomic caretaker, most notably in prevention of translocations and telomeric fusions. As Artemis deficiency is compatible with human life, Artemis may also suppress genomic instability in humans.
Figures



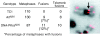
Comment in
-
VDJ recombination: Artemis and its in vivo role in hairpin opening.J Exp Med. 2003 Mar 3;197(5):543-7. doi: 10.1084/jem.20022210. J Exp Med. 2003. PMID: 12615895 Free PMC article. No abstract available.
References
-
- Ferguson, D.O., and F.W. Alt. 2001. DNA double strand break repair and chromosomal translocation: lessons from animal models. Oncogene. 20:5572–5579. - PubMed
-
- Jackson, S.P. 2002. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 23:687–696. - PubMed
-
- Bassing, C.H., W. Swat, and F.W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 109:S45–S55. - PubMed
-
- Bailey, S.M., M.N. Cornforth, A. Kurimasa, D.J. Chen, and E.H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science. 293:2462–2465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

