Components of the ligand for a Ni++ reactive human T cell clone
- PMID: 12615898
- PMCID: PMC2193829
- DOI: 10.1084/jem.20021762
Components of the ligand for a Ni++ reactive human T cell clone
Abstract
The major histocompatibility complex (MHC) restriction element for a human Ni(2+) reactive T cell, ANi-2.3, was identified as DR52c. A series of experiments established that the functional ligand for this T cell was a preformed complex of Ni(2+) bound to the combination of DR52c and a specific peptide that was generated in human and mouse B cells, but not in fibroblasts nor other antigen processing-deficient cells. In addition, ANi-2.3 recognition of this complex was dependent on His81 of the MHC beta chain, suggesting a role for this amino acid in Ni(2+) binding to MHC. We propose a general model for Ni(2+) recognition in which betaHis81 and two amino acids from the NH(2)-terminal part of the MHC bound peptide coordinate Ni(2+) which then interacts with some portion of the Valpha CDR1 or CDR2 region.
Figures
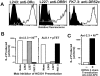





Comment in
-
Metal-derivatized major histocompatibility complex: zeroing in on contact hypersensitivity.J Exp Med. 2003 Mar 3;197(5):549-52. doi: 10.1084/jem.20022180. J Exp Med. 2003. PMID: 12615896 Free PMC article. No abstract available.
Similar articles
-
TCR reactivity in human nickel allergy indicates contacts with complementarity-determining region 3 but excludes superantigen-like recognition.J Immunol. 1999 Sep 1;163(5):2723-31. J Immunol. 1999. PMID: 10453014
-
A new type of metal recognition by human T cells: contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel.J Exp Med. 2003 May 19;197(10):1345-53. doi: 10.1084/jem.20030121. J Exp Med. 2003. PMID: 12756270 Free PMC article.
-
Metal-protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation.J Immunol. 2004 Feb 1;172(3):1926-34. doi: 10.4049/jimmunol.172.3.1926. J Immunol. 2004. PMID: 14734778
-
Diversity-oriented approaches for interrogating T-cell receptor repertoire, ligand recognition, and function.Immunol Rev. 2012 Nov;250(1):82-101. doi: 10.1111/imr.12006. Immunol Rev. 2012. PMID: 23046124 Free PMC article. Review.
-
Structural basis of metal hypersensitivity.Immunol Res. 2013 Mar;55(1-3):83-90. doi: 10.1007/s12026-012-8351-1. Immunol Res. 2013. PMID: 22983897 Free PMC article. Review.
Cited by
-
Identification of HLA-DRPhebeta47 as the susceptibility marker of hypersensitivity to beryllium in individuals lacking the berylliosis-associated supratypic marker HLA-DPGlubeta69.Respir Res. 2005 Aug 14;6(1):94. doi: 10.1186/1465-9921-6-94. Respir Res. 2005. PMID: 16098233 Free PMC article. Clinical Trial.
-
HLA-DR53 (DRB4∗01) associates with nickel sensitization.Ann Allergy Asthma Immunol. 2020 Nov;125(5):614-616. doi: 10.1016/j.anai.2020.07.011. Epub 2020 Jul 18. Ann Allergy Asthma Immunol. 2020. PMID: 32693207 Free PMC article. No abstract available.
-
Metal-derivatized major histocompatibility complex: zeroing in on contact hypersensitivity.J Exp Med. 2003 Mar 3;197(5):549-52. doi: 10.1084/jem.20022180. J Exp Med. 2003. PMID: 12615896 Free PMC article. No abstract available.
-
Palladium-Induced Temporal Internalization of MHC Class I Contributes to T Cell-Mediated Antigenicity.Front Immunol. 2021 Dec 23;12:736936. doi: 10.3389/fimmu.2021.736936. eCollection 2021. Front Immunol. 2021. PMID: 35003059 Free PMC article.
-
Using DR52c/Ni2+ mimotope tetramers to detect Ni2+ reactive CD4+ T cells in patients with joint replacement failure.Toxicol Appl Pharmacol. 2017 Sep 15;331:69-75. doi: 10.1016/j.taap.2017.05.020. Epub 2017 May 26. Toxicol Appl Pharmacol. 2017. PMID: 28554661 Free PMC article.
References
-
- Melian, A., E.M. Beckman, S.A. Porcelli, and M.B. Brenner. 1996. Antigen presentation by CD1 and MHC-encoded class I-like molecules. Curr. Opin. Immunol. 8:82–88. - PubMed
-
- Budinger, L., and M. Hertl. 2000. Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy. 55:108–115. - PubMed
-
- Hostynek, J.J. 1997. Gold: an allergen of growing significance. Food Chem. Toxicol. 35:839–844. - PubMed
-
- Zeng, Z., A.R. Castano, B.W. Segelke, E.A. Stura, P.A. Peterson, and I.A. Wilson. 1997. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 277:339–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

