Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen
- PMID: 12615900
- PMCID: PMC2193819
- DOI: 10.1084/jem.20021859
Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen
Abstract
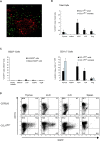
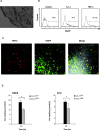
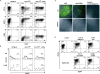
Dendritic cells (DCs)(*) fulfill an important regulatory function at the interface of the innate and adaptive immune system. The thymus and activation-regulated chemokine (TARC/CCL17) is produced by DCs and facilitates the attraction of activated T cells. Using a fluorescence-based in vivo reporter system, we show that CCL17 expression in mice is found in activated Langerhans cells and mature DCs located in various lymphoid and nonlymphoid organs, and is up-regulated after stimulation with Toll-like receptor ligands. DCs expressing CCL17 belong to the CD11b(+)CD8(-)Dec205(+) DC subset, including the myeloid-related DCs located in the subepithelial dome of Peyer's patches. CCL17-deficient mice mount diminished T cell-dependent contact hypersensitivity responses and display a deficiency in rejection of allogeneic organ transplants. In contrast to lymphoid organs located at external barriers of the skin and mucosa, CCL17 is not expressed in the spleen, even after systemic microbial challenge or after in vitro stimulation. These findings indicate that CCL17 production is a hallmark of local DC stimulation in peripheral organs but is absent from the spleen as a filter of blood-borne antigens.
Figures




References
-
- Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. - PubMed
-
- Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. - PubMed
-
- Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

