Characterization of the last subunit of the Arabidopsis COP9 signalosome: implications for the overall structure and origin of the complex
- PMID: 12615944
- PMCID: PMC150025
- DOI: 10.1105/tpc.009092
Characterization of the last subunit of the Arabidopsis COP9 signalosome: implications for the overall structure and origin of the complex
Abstract
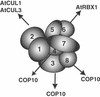
The COP9 signalosome (CSN) is an evolutionarily conserved protein complex that resembles the lid subcomplex of proteasomes. Through its ability to regulate specific proteasome-mediated protein degradation events, CSN controls multiple aspects of development. Here, we report the cloning and characterization of AtCSN2, the last uncharacterized CSN subunit from Arabidopsis. We show that the AtCSN2 gene corresponds to the previously identified FUS12 locus and that AtCSN2 copurifies with CSN, confirming that AtCSN2 is an integral component of CSN. AtCSN2 is not only able to interact with the SCF(TIR1) subunit AtCUL1, which is partially responsible for the regulatory interaction between CSN and SCF(TIR1), but also interacts with AtCUL3, suggesting that CSN is able to regulate the activity of other cullin-based E3 ligases through conserved interactions. Phylogenetic analysis indicated that the duplication and subsequent divergence events that led to the genes that encode CSN and lid subunits occurred before the divergence of unicellular and multicellular eukaryotic organisms and that the CSN subunits were more conserved than the lid subunits during evolution. Comparative analyses of the subunit interaction of CSN revealed a set of conserved subunit contacts and resulted in a model of CSN subunit topology, some aspects of which were substantiated by in vivo cross-link tests.
Figures

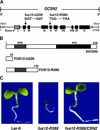

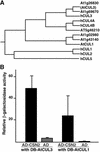
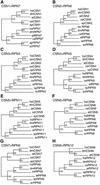

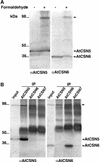
Comment in
-
Characterization of the last subunit of the Arabidopsis COP9 signalosome.Plant Cell. 2003 Mar;15(3):580-1. doi: 10.1105/tpc.150370. Plant Cell. 2003. PMID: 12615933 Free PMC article. No abstract available.
References
-
- Blatch, G.L., and Lassle, M. (1999). The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. Bioessays 21, 932–939. - PubMed
-
- Chamovitz, D.A., Wei, N., Osterlund, M.T., von Arnim, A.G., Staub, J.M., Matsui, M., and Deng, X.W. (1996). The COP9 complex, a novel multisubunit nuclear regulator involved in light control of a plant developmental switch. Cell 86, 115–121. - PubMed
-
- Cope, G.A., Suh, G.S., Aravind, L., Schwarz, S.E., Zipursky, S.L., Koonin, E.V., and Deshaies, R.J. (2002). Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of NEDD8 from CUL1. Science 298, 608–611. - PubMed
-
- Fraser, H.B., Hirsh, A.E., Steinmetz, L.M., Scharfe, C., and Feldman, M.W. (2002). Evolutionary rate in the protein interaction network. Science 296, 750–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

