Telomerase dependence of telomere lengthening in Ku80 mutant Arabidopsis
- PMID: 12615949
- PMCID: PMC150030
- DOI: 10.1105/tpc.008623
Telomerase dependence of telomere lengthening in Ku80 mutant Arabidopsis
Abstract
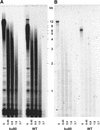
We have identified a ku80 mutant of Arabidopsis and show that telomerase is needed to generate the longer telomeres observed in this mutant. Telomeres are specialized nucleoprotein structures at the ends of chromosomes that permit cells to distinguish chromosome ends from double-strand breaks, thus preventing chromosome fusion events. Ku80 deficiency results in the lengthening of telomeres, a phenotype also seen in an Arabidopsis ku70 mutant. Furthermore, homogeneous populations of ku80 mutant cells show a steady increase in the length of telomere tracts, which reach an equilibrium length and then stabilize. In contrast to that in mammals, Ku80 deficiency in Arabidopsis cells does not cause end-to-end fusion of chromosomes. This telomere lengthening is dependent on the presence of telomerase, although it is not attributable to a significant increase in telomerase activity per se. These results demonstrate the essential role of the Ku80 protein as a negative regulator of telomerase function in plant cells.
Figures




References
-
- Bilaud, T., Brun, C., Ancelin, K., Koering, C.E., Laroche, T., and Gilson, E. (1997). Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17, 236–239. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

