Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart
- PMID: 12618230
- PMCID: PMC4332527
- DOI: 10.1016/s0008-6363(02)00725-3
Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart
Abstract
Objective and methods: In this study, we investigated whether Ca(2+) transients are altered in Purkinje cell aggregates dispersed from the subendocardium overlying the infarcted zone of the left ventricle (IZPCs) 48 h after coronary artery occlusion. To do so, we combined epifluorescent imaging with microelectrode recordings of IZPCs and normal canine Purkinje cell aggregates (NZPCs).
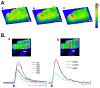
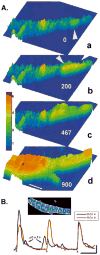
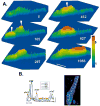
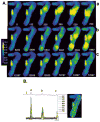
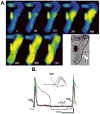
Results: NZPCs respond to an action potential (AP) by a small Ca(2+) transient at the cell surface immediately after the AP upstroke followed by a large [Ca(2+)] transient, which propagates to the cell core. In addition, focal Ca(2+) waves can originate spontaneously later during the AP or during the diastolic interval (Circ Res 2000;86:448-55) and then propagate throughout the aggregate as 'cell-wide Ca(2+) waves'. Electrically-evoked Ca(2+) transients in IZPCs arose significantly faster than those in NZPCs, and showed substantial spatiotemporal nonuniformity within an IZPC aggregate as well as between IZPC aggregates. IZPCs showed, hitherto undetected, low amplitude, micro Ca(2+) transients (extent <or=5 microm) at a fivefold higher incidence than in NZPCs. Micro Ca(2+) transients appeared to meander over distances <or=100 microm and reduced the local Ca(2+) transient of the next paced beat. Micro Ca(2+) transients nearly always preceded the cell-wide Ca(2+)waves, which occurred more frequently in IZPCs than in NZPCs and caused non-driven electrical activity of the Purkinje aggregate.
Conclusions: Micro Ca(2+) transients preceded cell-wide Ca(2+) waves so often that it is probable that micro Ca(2+) transients induced cell-wide Ca(2+) waves. Cell-wide Ca(2+) waves, in turn, clearly elicited spontaneous APs. We propose that the high incidence of micro Ca(2+) transients in IZPCs is a fundamental element of the abnormal Ca(2+) handling of diseased Purkinje cells, underlying arrhythmias originating in the subendocardial Purkinje network post myocardial infarction.
Figures


References
-
- Janse MJ, Wit Al. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. - PubMed
-
- Pinto JMB, Boyden PA. Electrophysiologic remodeling in ischemia and infarction. Cardiovasc Res. 1999;42:284–297. - PubMed
-
- Harris AS. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–1328. - PubMed
-
- Boyden PA, Albala A, Dresdner K. Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24 hour infarcted hearts. Circ Res. 1989;65:955–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

