Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum
- PMID: 12618456
- PMCID: PMC150123
- DOI: 10.1128/JB.185.6.1923-1934.2003
Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum
Erratum in
- J Bacteriol. 2003 May;185(9):2973
Abstract
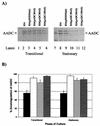
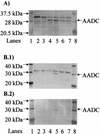
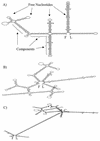
We investigated the effect of antisense RNA (asRNA) structural properties on the downregulation efficacy of enzymes in the acetone-formation pathway (acetoacetate decarboxylase [AADC] and coenzyme A-transferase [CoAT]) of Clostridium acetobutylicum strain ATCC 824. First, we generated three strains, C. acetobutylicum ATCC 824 (pADC38AS), 824(pADC68AS), and 824(pADC100AS), which contain plasmids that produce asRNAs of various lengths against the AADC (adc) transcript. Western analysis showed that all three strains exhibit low levels of AADC compared to the plasmid control [ATCC 824(pSOS95del)]. By using computational algorithms, the three different asRNAs directed toward AADC, along with previously reported clostridial asRNAs, were examined for structural features (free nucleotides and components). When the normalized metrics of these structural features were plotted against percent downregulation, only the component/nucleotide ratio correlated well with in vivo asRNA effectiveness. Despite the significant downregulation of AADC in these strains, there were no concomitant effects on acetone formation. These findings suggest that AADC does not limit acetone formation and, thus, we targeted next the CoAT. Using the component/nucleotide ratio as a selection parameter, we developed three strains [ATCC 824 (pCTFA2AS), 824(pCTFB1AS), and 824(pCOAT11AS)] which express asRNAs to downregulate either or both of the CoAT subunits. Compared to the plasmid control strain, these strains produced substantially low levels of acetone and butanol and Western blot analyses showed significantly low levels of both CoAT subunits. These results show that CoAT is the rate-limiting enzyme in acetone formation and strengthen the hypothesis that the component/nucleotide ratio is a predictive indicator of asRNA effectiveness.
Figures
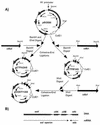

 , adc100-asRNA; ♦, ctfb1-asRNA; (⋄), coat11-asRNA-b.
, adc100-asRNA; ♦, ctfb1-asRNA; (⋄), coat11-asRNA-b.
References
-
- Berghammer, H., and B. Auer. 1993. “Easypreps”: fast and easy plasmid minipreparation for analysis of recombinant clones in E. coli. BioTechniques 14:524-528. - PubMed
-
- Buday, Z., J. C. Linden, and M. N. Karim. 1990. Improved acetone-butanol fermentation analysis using subambient HPLC column temperature. Enzyme Microb. Technol. 12:24-27.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

