Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle
- PMID: 12618515
- PMCID: PMC151900
- DOI: 10.1172/JCI17038
Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle
Abstract
Our purpose here is to test the hypothesis that Randall's plaques, calcium phosphate deposits in kidneys of patients with calcium renal stones, arise in unique anatomical regions of the kidney, their formation conditioned by specific stone-forming pathophysiologies. To test this hypothesis, we performed intraoperative biopsies of plaques in kidneys of idiopathic-calcium-stone formers and patients with stones due to obesity-related bypass procedures and obtained papillary specimens from non-stone formers after nephrectomy. Plaque originates in the basement membranes of the thin loops of Henle and spreads from there through the interstitium to beneath the urothelium. Patients who have undergone bypass surgery do not produce such plaque but instead form intratubular hydroxyapatite crystals in collecting ducts. Non-stone formers also do not form plaque. Plaque is specific to certain kinds of stone-forming patients and is initiated specifically in thin-limb basement membranes by mechanisms that remain to be elucidated.
Figures

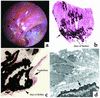


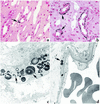



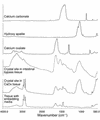

Comment in
-
Nephrolithiasis: site of the initial solid phase.J Clin Invest. 2003 Mar;111(5):602-5. doi: 10.1172/JCI18016. J Clin Invest. 2003. PMID: 12618514 Free PMC article. No abstract available.
References
-
- Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N. Engl. J. Med. 1979;300:337–340. - PubMed
-
- Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J. Urol. 1997;158:2062–2064. - PubMed
-
- Randall R. Papillary pathology as precursor of primary renal calculus. J. Urol. 1940;44:580–589.
-
- Mandel, G.S., and Mandel, N.S. 1996. Crystal-crystal interactions. In Kidney stones: medical and surgical management. F. L. Coe, M. J. Favus, C. Y. C. Pak, J. H. Parks, and G. M. Preminger, editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 115–128.

