Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases
- PMID: 12618525
- PMCID: PMC151905
- DOI: 10.1172/JCI17423
Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases
Abstract
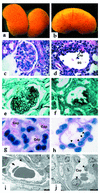
Kidney disease affects over 20 million people in the United States alone. Although the causes of renal failure are diverse, the glomerular filtration barrier is often the target of injury. Dysregulation of VEGF expression within the glomerulus has been demonstrated in a wide range of primary and acquired renal diseases, although the significance of these changes is unknown. In the glomerulus, VEGF-A is highly expressed in podocytes that make up a major portion of the barrier between the blood and urinary spaces. In this paper, we show that glomerular-selective deletion or overexpression of VEGF-A leads to glomerular disease in mice. Podocyte-specific heterozygosity for VEGF-A resulted in renal disease by 2.5 weeks of age, characterized by proteinuria and endotheliosis, the renal lesion seen in preeclampsia. Homozygous deletion of VEGF-A in glomeruli resulted in perinatal lethality. Mutant kidneys failed to develop a filtration barrier due to defects in endothelial cell migration, differentiation, and survival. In contrast, podocyte-specific overexpression of the VEGF-164 isoform led to a striking collapsing glomerulopathy, the lesion seen in HIV-associated nephropathy. Our data demonstrate that tight regulation of VEGF-A signaling is critical for establishment and maintenance of the glomerular filtration barrier and strongly supports a pivotal role for VEGF-A in renal disease.
Figures





Comment in
-
Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered?J Clin Invest. 2003 Mar;111(5):600-2. doi: 10.1172/JCI18015. J Clin Invest. 2003. PMID: 12618513 Free PMC article. No abstract available.
References
-
- Mundel P, Reiser J. New aspects of podocyte cell biology. Kidney Blood Press. Res. 1997;20:173–176. - PubMed
-
- Abrahamson DR. Glomerulogenesis in the developing kidney. Semin. Nephrol. 1991;11:375–389. - PubMed
-
- Kriz W, Lemley K. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 1999;8:489–497. - PubMed
-
- Kriz W. Evolving role of the podocyte in chronic renal failure. Kidney Blood Press. Res. 1997;20:180–183. - PubMed
-
- Shirato I, et al. The development of focal segmental glomerulosclerosis in masugi nephritis is based on progressive podocyte damage. Virchows Arch. 1996;429:255–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

