VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema
- PMID: 12618526
- PMCID: PMC151891
- DOI: 10.1172/JCI15830
VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema
Abstract
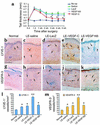
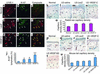
Although lymphedema is a common clinical condition, treatment for this disabling condition remains limited and largely ineffective. Recently, it has been reported that overexpression of VEGF-C correlates with increased lymphatic vessel growth (lymphangiogenesis). However, the effect of VEGF-C-induced lymphangiogenesis on lymphedema has yet to be demonstrated. Here we investigated the impact of local transfer of naked plasmid DNA encoding human VEGF-C (phVEGF-C) on two animal models of lymphedema: one in the rabbit ear and the other in the mouse tail. In a rabbit model, following local phVEGF-C gene transfer, VEGFR-3 expression was significantly increased. This gene transfer led to a decrease in thickness and volume of lymphedema, improvement of lymphatic function demonstrated by serial lymphoscintigraphy, and finally, attenuation of the fibrofatty changes of the skin, the final consequences of lymphedema. The favorable effect of phVEGF-C on lymphedema was reconfirmed in a mouse tail model. Immunohistochemical analysis using lymphatic-specific markers: VEGFR-3, lymphatic endothelial hyaluronan receptor-1, together with the proliferation marker Ki-67 Ab revealed that phVEGF-C transfection potently induced new lymphatic vessel growth. This study, we believe for the first time, documents that gene transfer of phVEGF-C resolves lymphedema through direct augmentation of lymphangiogenesis. This novel therapeutic strategy may merit clinical investigation in patients with lymphedema.
Figures



References
-
- Browse NL. The diagnosis and management of primary lymphedema. J. Vasc. Surg. 1986;3:181–184. - PubMed
-
- de Almeida AB, Freedman DO. Epidemiology and immunopathology of bancroftian filariasis. Microbes Infect. 1999;1:1015–1022. - PubMed
-
- Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc. Med. 1998;3:145–156. - PubMed
-
- Ko DS, Lerner R, Klose G, Cosimi AB. Effective treatment of lymphedema of the extremities. Arch. Surg. 1998;133:452–458. - PubMed
-
- Slavin SA, Upton J, Kaplan WD, Van den Abbeele AD. An investigation of lymphatic function following free-tissue transfer. Plast. Reconstr. Surg. 1997;99:730–741; discussion 742–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

