CloQ, a prenyltransferase involved in clorobiocin biosynthesis
- PMID: 12618544
- PMCID: PMC151338
- DOI: 10.1073/pnas.0337708100
CloQ, a prenyltransferase involved in clorobiocin biosynthesis
Abstract
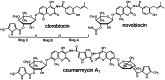
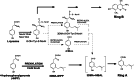
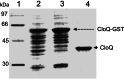
Ring A (3-dimethylallyl-4-hydroxybenzoic acid) is a structural moiety of the aminocoumarin antibiotics novobiocin and clorobiocin. In the present study, the prenyltransferase involved in the biosynthesis of this moiety was identified from the clorobiocin producer (Streptomyces roseochromogenes), overexpressed, and purified. It is a soluble, monomeric 35-kDa protein, encoded by the structural gene cloQ. 4-Hydroxyphenylpyruvate and dimethylallyl diphosphate were identified as the substrates of this enzyme, with K(m) values determined as 25 and 35 microM, respectively. A gene inactivation experiment confirmed that cloQ is essential for ring A biosynthesis. Database searches did not reveal any similarity of CloQ to known prenyltransferases, and the enzyme did not contain the typical prenyl diphosphate binding site (N/D)DXXD. In contrast to most of the known prenyltransferases, the enzymatic activity was not dependent on the presence of magnesium, and in contrast to the membrane-bound polyprenyltransferases involved in ubiquinone biosynthesis, CloQ did not accept 4-hydroxybenzoic acid as substrate. CloQ and the similar NovQ from the novobiocin producer seem to belong to a new class of prenyltransferases.
Figures


References
-
- Gormley N A, Orphanides G, Meyer A, Cullis P M, Maxwell A. Biochemistry. 1996;35:5083–5092. - PubMed
-
- Berger J, Batcho A D. J Chromatogr Lib. 1978;15:101–159.
-
- Lanoot B, Vancanneyt M, Cleenwerck I, Wang L, Li W, Liu Z, Swings J. Int J Syst Evol Microbiol. 2002;52:823–829. - PubMed
-
- Li S-M, Hennig S, Heide L. Tetrahedron Lett. 1998;39:2717–2720.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

