The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers
- PMID: 12620104
- PMCID: PMC153459
- DOI: 10.1186/gb-2003-4-3-r19
The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers
Abstract
Background: The rhomboid family of polytopic membrane proteins shows a level of evolutionary conservation unique among membrane proteins. They are present in nearly all the sequenced genomes of archaea, bacteria and eukaryotes, with the exception of several species with small genomes. On the basis of experimental studies with the developmental regulator rhomboid from Drosophila and the AarA protein from the bacterium Providencia stuartii, the rhomboids are thought to be intramembrane serine proteases whose signaling function is conserved in eukaryotes and prokaryotes.
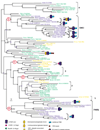
Results: Phylogenetic tree analysis carried out using several independent methods for tree constructions and the corresponding statistical tests suggests that, despite its broad distribution in all three superkingdoms, the rhomboid family was not present in the last universal common ancestor of extant life forms. Instead, we propose that rhomboids evolved in bacteria and have been acquired by archaea and eukaryotes through several independent horizontal gene transfers. In eukaryotes, two distinct, ancient acquisitions apparently gave rise to the two major subfamilies, typified by rhomboid and PARL (presenilins-associated rhomboid-like protein), respectively. Subsequent evolution of the rhomboid family in eukaryotes proceeded by multiple duplications and functional diversification through the addition of extra transmembrane helices and other domains in different orientations relative to the conserved core that harbors the protease activity.
Conclusions: Although the near-universal presence of the rhomboid family in bacteria, archaea and eukaryotes appears to suggest that this protein is part of the heritage of the last universal common ancestor, phylogenetic tree analysis indicates a likely bacterial origin with subsequent dissemination by horizontal gene transfer. This emphasizes the importance of explicit phylogenetic analysis for the reconstruction of ancestral life forms. A hypothetical scenario for the origin of intracellular membrane proteases from membrane transporters is proposed.
Figures


Similar articles
-
Bioinformatics perspective on rhomboid intramembrane protease evolution and function.Biochim Biophys Acta. 2013 Dec;1828(12):2937-43. doi: 10.1016/j.bbamem.2013.06.031. Epub 2013 Jul 8. Biochim Biophys Acta. 2013. PMID: 23845876 Free PMC article. Review.
-
Conservation of intramembrane proteolytic activity and substrate specificity in prokaryotic and eukaryotic rhomboids.Curr Biol. 2002 Sep 3;12(17):1507-12. doi: 10.1016/s0960-9822(02)01092-8. Curr Biol. 2002. PMID: 12225666
-
Algorithms for computing parsimonious evolutionary scenarios for genome evolution, the last universal common ancestor and dominance of horizontal gene transfer in the evolution of prokaryotes.BMC Evol Biol. 2003 Jan 6;3:2. doi: 10.1186/1471-2148-3-2. Epub 2003 Jan 6. BMC Evol Biol. 2003. PMID: 12515582 Free PMC article.
-
Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases.Genome Res. 2007 Nov;17(11):1634-46. doi: 10.1101/gr.6425307. Epub 2007 Oct 15. Genome Res. 2007. PMID: 17938163 Free PMC article.
-
Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature.Trends Parasitol. 2005 Jun;21(6):254-8. doi: 10.1016/j.pt.2005.04.009. Trends Parasitol. 2005. PMID: 15922242 Review.
Cited by
-
Cross genome comparisons of serine proteases in Arabidopsis and rice.BMC Genomics. 2006 Aug 9;7:200. doi: 10.1186/1471-2164-7-200. BMC Genomics. 2006. PMID: 16895613 Free PMC article.
-
Mechanism of intramembrane proteolysis investigated with purified rhomboid proteases.EMBO J. 2005 Feb 9;24(3):464-72. doi: 10.1038/sj.emboj.7600537. Epub 2004 Dec 23. EMBO J. 2005. PMID: 15616571 Free PMC article.
-
PARL Leu262Val is not associated with fasting insulin levels in UK populations.Diabetologia. 2006 Nov;49(11):2649-52. doi: 10.1007/s00125-006-0443-9. Epub 2006 Sep 21. Diabetologia. 2006. PMID: 17019603 Free PMC article.
-
Activity-based probes for rhomboid proteases discovered in a mass spectrometry-based assay.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2472-7. doi: 10.1073/pnas.1215076110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359682 Free PMC article.
-
Bioinformatics perspective on rhomboid intramembrane protease evolution and function.Biochim Biophys Acta. 2013 Dec;1828(12):2937-43. doi: 10.1016/j.bbamem.2013.06.031. Epub 2013 Jul 8. Biochim Biophys Acta. 2013. PMID: 23845876 Free PMC article. Review.
References
-
- Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, Rao BS, Kiryutin B, Galperin MY, Fedorova ND, Koonin EV. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29:22–28. doi: 10.1093/nar/29.1.22. - DOI - PMC - PubMed
-
- Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 1993;7:961–973. - PubMed
-
- Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, Howard K, Bier E. rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development. 1999;126:2663–2676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

