Electricity production by Geobacter sulfurreducens attached to electrodes
- PMID: 12620842
- PMCID: PMC150094
- DOI: 10.1128/AEM.69.3.1548-1555.2003
Electricity production by Geobacter sulfurreducens attached to electrodes
Abstract
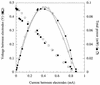
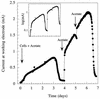
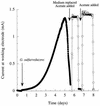
Previous studies have suggested that members of the Geobacteraceae can use electrodes as electron acceptors for anaerobic respiration. In order to better understand this electron transfer process for energy production, Geobacter sulfurreducens was inoculated into chambers in which a graphite electrode served as the sole electron acceptor and acetate or hydrogen was the electron donor. The electron-accepting electrodes were maintained at oxidizing potentials by connecting them to similar electrodes in oxygenated medium (fuel cells) or to potentiostats that poised electrodes at +0.2 V versus an Ag/AgCl reference electrode (poised potential). When a small inoculum of G. sulfurreducens was introduced into electrode-containing chambers, electrical current production was dependent upon oxidation of acetate to carbon dioxide and increased exponentially, indicating for the first time that electrode reduction supported the growth of this organism. When the medium was replaced with an anaerobic buffer lacking nutrients required for growth, acetate-dependent electrical current production was unaffected and cells attached to these electrodes continued to generate electrical current for weeks. This represents the first report of microbial electricity production solely by cells attached to an electrode. Electrode-attached cells completely oxidized acetate to levels below detection (<10 micro M), and hydrogen was metabolized to a threshold of 3 Pa. The rates of electron transfer to electrodes (0.21 to 1.2 micro mol of electrons/mg of protein/min) were similar to those observed for respiration with Fe(III) citrate as the electron acceptor (E(o)' =+0.37 V). The production of current in microbial fuel cell (65 mA/m(2) of electrode surface) or poised-potential (163 to 1,143 mA/m(2)) mode was greater than what has been reported for other microbial systems, even those that employed higher cell densities and electron-shuttling compounds. Since acetate was completely oxidized, the efficiency of conversion of organic electron donor to electricity was significantly higher than in previously described microbial fuel cells. These results suggest that the effectiveness of microbial fuel cells can be increased with organisms such as G. sulfurreducens that can attach to electrodes and remain viable for long periods of time while completely oxidizing organic substrates with quantitative transfer of electrons to an electrode.
Figures




References
-
- Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms harvesting energy from marine sediments. Science 295:483-485. - PubMed
-
- Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens access Fe(III) oxide by chemotaxis. Nature 416:767-769. - PubMed
-
- Choi, Y., J. Song, S. Jung, and S. Kim. 2001. Optimization of the performance of microbial fuel cells containing alkalophilic Bacillus sp. J. Microbiol. Biotechnol. 11:863-869.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

