Cost-effectiveness analysis of the gen-probe amplified mycobacterium tuberculosis direct test as used routinely on smear-positive respiratory specimens
- PMID: 12624014
- PMCID: PMC150318
- DOI: 10.1128/JCM.41.3.948-953.2003
Cost-effectiveness analysis of the gen-probe amplified mycobacterium tuberculosis direct test as used routinely on smear-positive respiratory specimens
Abstract
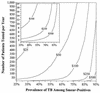
A decision analysis was conducted to evaluate the cost-effectiveness of programs in which the Amplified Mycobacterium Tuberculosis Direct test (MTD) (Gen-Probe) is used to rapidly exclude Mycobacterium tuberculosis complex as a cause of disease in smear-positive respiratory specimens. MTD sensitivity, specificity, and probability of inhibition for smear-positive specimens were estimated from literature reports. Costs and laboratory performance characteristics were determined from review of records and practices at an urban hospital in the mid-Atlantic United States. In the base case, 31.4% of smear-positive specimens were assumed to be culture positive for M. tuberculosis. Under these conditions, the marginal cost of the MTD testing program was estimated as $338 per smear-positive patient, or $494 per early exclusion of tuberculosis based on negative MTD results. By comparison, the cost of respiratory isolation ($27.77/day) and drugs ($5.66/day) averted by MTD testing was estimated at $201 per early tuberculosis exclusion. MTD testing was therefore not cost-effective in this scenario. Sensitivity analysis revealed that cost-effectiveness estimates are sensitive to the number of smear-positive specimens processed annually, the relative prevalence of M. tuberculosis in smear-positive specimens, and the marginal daily cost of respiratory isolation. A decision tool is therefore presented for assessing the cost-effectiveness of MTD under various combinations of those three variables. While routine MTD testing of smear-positive specimens is not expected to be cost-saving for most individual hospitals, centralized reference laboratories may be able to implement MTD in a cost-effective manner across a wide range of situations.
Figures
References
-
- American Thoracic Society Workshop. 1997. Rapid diagnostic tests for tuberculosis: what is the appropriate use? Am. J. Respir. Crit. Care Med. 155:1804-1814. - PubMed
-
- Catanzaro, A., S. Perry, J. E. Clarridge, S. Dunbar, S. Goodnight-White, P. A. LoBue, C. Peter, G. E. Pfyffer, M. F. Sierra, R. Weber, G. Woods, G. Mathews, V. Jonas, K. Smith, and P. Della-Latta. 2000. The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis: results of a multicenter prospective trial. JAMA 283:639-645. - PubMed
-
- Centers for Disease Control and Prevention. 1994. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. Morb. Mortal. Wkly. Rep. 43(RR-13):1-132. - PubMed
-
- Centers for Disease Control and Prevention. 2000. Update: nucleic acid amplification tests for tuberculosis. Morb. Mortal. Wkly. Rep. 49:593-594. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

