High-level expression and purification of a truncated merozoite antigen-2 of Babesia equi in Escherichia coli and its potential for immunodiagnosis
- PMID: 12624044
- PMCID: PMC150322
- DOI: 10.1128/JCM.41.3.1147-1151.2003
High-level expression and purification of a truncated merozoite antigen-2 of Babesia equi in Escherichia coli and its potential for immunodiagnosis
Abstract
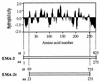
The gene encoding a truncated merozoite antigen-2 (EMA-2t) of Babesia equi was cloned and highly expressed in Escherichia coli as a glutathione S-transferase fusion protein (G-rEMA-2t). Both G-rEMA-2t and rEMA-2t (after the removal of glutathione S-transferase) had good antigenicity. Either Western blot analysis with rEMA-2t or enzyme-linked immunosorbent assay (ELISA) with G-rEMA-2t clearly discriminated the sera of horses experimentally infected with B. equi from sera of horses infected with Babesia caballi and healthy horses, although rEMA-2t was not suitable for ELISA, probably owing to its poor absorbability to the plates. The specific antibodies in B. equi-infected horses were detectable during both acute and latent infection (6 to 244 days postinfection). Horse sera from Jilin Province, China, were examined by the two tests. The seroprevalence of B. equi was 49.2% (31 of 63 sera) by Western blot analysis with rEMA-2t and 47.6% (30 of 63 sera) by ELISA with G-rEMA-2t. The correspondence was 98.4% (62 of 63 sera) between the two tests. The results indicate that G-rEMA-2t and rEMA-2t proteins should be suitable antigens for the development of an effective immunodiagnostic assay due to their high sensitivity, specificity, and great yield.
Figures



References
-
- Avarazed, A., D. T. de Waal, I. Igarashi, A. Saito, T. Oyamada, Y. Toyoda, and N. Suzuki. 1997. Prevalence of equine piroplasmosis in central Mongolia. Onderstepoort J. Vet. Res. 64:141-145. - PubMed
-
- Avarazed, A., I. Igarashi, D. T. DeWaal, S. Kawai, Y. Oomori, N. Inoue, Y. Maki, Y. Omata, A. Saito, H. Nagasawa, Y. Toyoda, and N. Suzuki. 1998. Monoclonal antibody against Babesia equi: characterization and potential application of antigen for serodiagnosis. J. Clin. Microbiol. 36:1835-1839. - PMC - PubMed
-
- Bruning, A. 1996. Equine piroplasmosis: an update on diagnosis, treatment, and prevention. Br. Vet. J. 152:139-151. - PubMed
-
- Burg, J. D., D. Perelmam, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 41:3584-3591. - PubMed
-
- Chen, X., Y. Gong, H. Li, Z. Lun, and M. Fung. 2001. High-level expression and purification of immunogenic recombinant SAG1 (P30) of Toxoplasma gondii in Escherichia coli. Protein Express. Purif. 23:33-37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

