Increasing vitamin C content of plants through enhanced ascorbate recycling
- PMID: 12624189
- PMCID: PMC152326
- DOI: 10.1073/pnas.0635176100
Increasing vitamin C content of plants through enhanced ascorbate recycling
Abstract
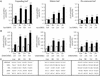
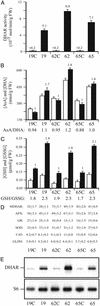
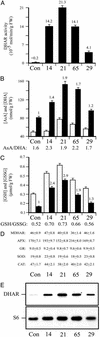
Vitamin C (ascorbic acid) is essential to prevent disease associated with connective tissue (e.g., scurvy), improves cardiovascular and immune cell functions, and is used to regenerate alpha-tocopherol (vitamin E). In contrast to most animals, humans lack the ability to synthesize ascorbic acid as a result of a mutation in the last enzyme required for ascorbate biosynthesis. Vitamin C, therefore, must be obtained from dietary sources and, because it cannot be stored in the body, it must be obtained regularly. Once used, ascorbic acid can be regenerated from its oxidized form in a reaction catalyzed by dehydroascorbate reductase (DHAR). To examine whether overexpression of DHAR in plants would increase the level of ascorbic acid through improved ascorbate recycling, a DHAR cDNA from wheat was isolated and expressed in tobacco and maize, where DHAR expression was increased up to 32- and 100-fold, respectively. The increase in DHAR expression increased foliar and kernel ascorbic acid levels 2- to 4-fold and significantly increased the ascorbate redox state in both tobacco and maize. In addition, the level of glutathione, the reductant used by DHAR, also increased, as did its redox state. These results demonstrate that the vitamin C content of plants can be elevated by increasing expression of the enzyme responsible for recycling ascorbate.
Figures

References
-
- Wheeler G L, Jones M A, Smirnoff N. Nature. 1998;393:365–369. - PubMed
-
- Smirnoff N, Wheeler G L. Crit Rev Biochem Mol Biol. 2000;35:291–314. - PubMed
-
- Smirnoff N, Conklin P L, Loewus F A. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:437–467. - PubMed
-
- Asada K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. - PubMed
-
- Smirnoff N. Curr Opin Plant Biol. 2000;3:229–235. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

