5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle
- PMID: 12626670
- PMCID: PMC2342869
- DOI: 10.1113/jphysiol.2002.037952
5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle
Abstract
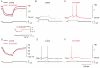
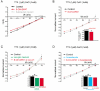
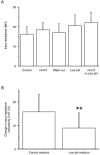
The modulatory effects of serotonin mediated by 5-HT1A receptors in adult spinal motoneurons were investigated by intracellular recordings in a slice preparation from the turtle. In current-clamp mode, activation of 5-HT1A receptors by 8-OH-DPAT led to depolarization and an increase in input resistance in most motoneurons but caused hyperpolarization and a decrease in input resistance in the remaining smaller fraction of cells. When slices were preincubated in medium containing the 5-HT1A receptor antagonist WAY-100635, 8-OH-DPAT had no effect. In voltage-clamp mode, with 1 mM CsCl in the bathing medium, 8-OH-DPAT consistently inhibited a leak current that was sensitive to extracellular acidification and anandamide, a TASK-1 channel blocker. In medium with a low pH, as in the presence of anandamide, 8-OH-DPAT had no effect. Our results show that activation of 5-HT1A receptors contributes to the excitatory effect of serotonin on spinal motoneurons by inhibition of a TASK-1 potassium channel leading to depolarization and increased input resistance.
Figures


Similar articles
-
5-HT inhibits N-type but not L-type Ca(2+) channels via 5-HT1A receptors in lamprey spinal neurons.Eur J Neurosci. 2003 Dec;18(11):2919-24. doi: 10.1111/j.1460-9568.2003.03051.x. Eur J Neurosci. 2003. PMID: 14656287
-
In vivo evidence for serotonin 5-HT2C receptor-mediated long-lasting excitability of lumbar spinal reflex and its functional interaction with 5-HT1A receptor in the mammalian spinal cord.Brain Res Bull. 2008 Mar 28;75(5):674-80. doi: 10.1016/j.brainresbull.2007.11.003. Epub 2007 Nov 29. Brain Res Bull. 2008. PMID: 18355645
-
Voltage-dependent calcium currents in trigeminal motoneurons of early postnatal rats: modulation by 5-HT receptors.J Neurophysiol. 2005 Sep;94(3):2063-72. doi: 10.1152/jn.00178.2005. Epub 2005 Jun 22. J Neurophysiol. 2005. PMID: 15972834
-
Modulation of motoneuron activity by serotonin.Dan Med J. 2016 Feb;63(2):B5204. Dan Med J. 2016. PMID: 26836802 Review.
-
Pharmacology of neuronal background potassium channels.Neuropharmacology. 2003 Jan;44(1):1-7. doi: 10.1016/s0028-3908(02)00339-8. Neuropharmacology. 2003. PMID: 12559116 Review.
Cited by
-
Anxiolytics may promote locomotor function recovery in spinal cord injury patients.Neuropsychiatr Dis Treat. 2008 Aug;4(4):759-63. doi: 10.2147/ndt.s2839. Neuropsychiatr Dis Treat. 2008. PMID: 19043520 Free PMC article.
-
Neuromodulation of neurons and synapses.Curr Opin Neurobiol. 2014 Dec;29:48-56. doi: 10.1016/j.conb.2014.05.003. Epub 2014 Jun 5. Curr Opin Neurobiol. 2014. PMID: 24907657 Free PMC article. Review.
-
Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation.Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4774-9. doi: 10.1073/pnas.1216150110. Epub 2013 Mar 4. Proc Natl Acad Sci U S A. 2013. PMID: 23487756 Free PMC article.
-
General Principles of Neuronal Co-transmission: Insights From Multiple Model Systems.Front Neural Circuits. 2019 Jan 21;12:117. doi: 10.3389/fncir.2018.00117. eCollection 2018. Front Neural Circuits. 2019. PMID: 30728768 Free PMC article. Review.
-
Modeling the mammalian locomotor CPG: insights from mistakes and perturbations.Prog Brain Res. 2007;165:235-53. doi: 10.1016/S0079-6123(06)65015-2. Prog Brain Res. 2007. PMID: 17925250 Free PMC article. Review.
References
-
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. - PubMed
-
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. - PubMed
-
- Hille B. Ionic Channels of Excitable Membranes. 3. MA USA: Blackwell Science Inc; 2001.
-
- Ho BY, Uezono Y, Takada S, Takase I, Izumi F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Receptors Channels. 1999;6:363–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

