The human respiratory gate
- PMID: 12626671
- PMCID: PMC2342859
- DOI: 10.1113/jphysiol.2002.037192
The human respiratory gate
Abstract
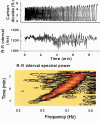
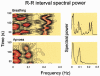
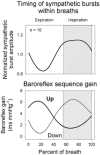
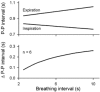
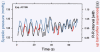
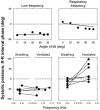
Respiratory activity phasically alters membrane potentials of preganglionic vagal and sympathetic motoneurones and continuously modulates their responsiveness to stimulatory inputs. The most obvious manifestation of this 'respiratory gating' is respiratory sinus arrhythmia, the rhythmic fluctuations of electrocardiographic R-R intervals observed in healthy resting humans. Phasic autonomic motoneurone firing, reflecting the throughput of the system, depends importantly on the intensity of stimulatory inputs, such that when levels of stimulation are low (as with high arterial pressure and sympathetic activity, or low arterial pressure and vagal activity), respiratory fluctuations of sympathetic or vagal firing are also low. The respiratory gate has a finite capacity, and high levels of stimulation override the ability of respiration to gate autonomic responsiveness. Autonomic throughput also depends importantly on other factors, including especially, the frequency of breathing, the rate at which the gate opens and closes. Respiratory sinus arrhythmia is small at rapid, and large at slow breathing rates. The strong correlation between systolic pressure and R-R intervals at respiratory frequencies reflects the influence of respiration on these two measures, rather than arterial baroreflex physiology. A wide range of evidence suggests that respiratory activity gates the timing of autonomic motoneurone firing, but does not influence its tonic level. I propose that the most enduring significance of respiratory gating is its use as a precisely controlled experimental tool to tease out and better understand otherwise inaccessible human autonomic neurophysiological mechanisms.
Figures




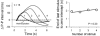



References
-
- Angelone A, Coulter NA., Jr Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol. 1964;19:479–482. - PubMed
-
- Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate. I-The reflex mechanism of the respiratory arrhythmia. Proc R Soc Lond B Biol Sci. 1936a;119 B:191–217.
-
- Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate. II-The central mechanism of the respiratory arrhythmia and the inter-relations between the central and the reflex mechanisms. Proc R Soc Lond B Biol Sci. 1936b;119 B:218–230.
-
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–2688. - PubMed
-
- Baselli G, Cerutti S, Badilini F, Biancardi L, Porta A, Pagani M, Lombardi F, Rimoldi O, Furlan R, Malliani A. Model for the assessment of heart period and arterial pressure variability interactions and respiration influences. Med Biol Eng Comput. 1994;32:143–152. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

