Human mitochondrial DNA is packaged with TFAM
- PMID: 12626705
- PMCID: PMC152855
- DOI: 10.1093/nar/gkg251
Human mitochondrial DNA is packaged with TFAM
Abstract
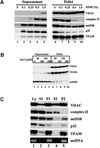
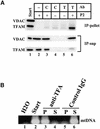
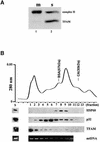
Mitochondrial transcription factor A (TFAM), a member of the high mobility group proteins, is essential for maintenance of mitochondrial DNA (mtDNA). Most TFAM and mtDNA (both of which are normally soluble) was recovered from the particulate fraction of human placental mitochondria when extracted with the non-ionic detergent Nonidet P-40. mtDNA and TFAM were co-immunoprecipitated by anti-TFAM antibodies. TFAM was released into the supernatant by DNase I digestion of mtDNA in the particulate fraction. Thus, TFAM and mtDNA are tightly associated with each other, and it is likely that few TFAM or mtDNA molecules exist in an unbound form in mitochondria. Based on the fact that TFAM is abundant enough to wrap mtDNA entirely, these results suggest that human mtDNA is packaged with TFAM.
Figures


References
-
- Falkenberg M., Gaspari,M., Rantanen,A., Trifunovic,A., Larsson,N.G. and Gustafsson,C.M. (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nature Genet., 31, 289–294. - PubMed
-
- Shadel G.S. and Clayton,D.A. (1997) Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem., 66, 409–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

