Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA
- PMID: 12626738
- PMCID: PMC152250
- DOI: 10.1073/pnas.0630422100
Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA
Abstract
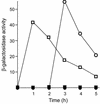
Modulation of the structure of a leader RNA to control formation of an intrinsic termination signal is a common mechanism for regulation of gene expression in bacteria. Expression of the S box genes in Gram-positive organisms is induced in response to limitation for methionine. We previously postulated that methionine availability is monitored by binding of a regulatory factor to the leader RNA and suggested that methionine or S-adenosylmethionine (SAM) could serve as the metabolic signal. In this study, we show that efficient termination of the S box leader region by bacterial RNA polymerase depends on SAM but not on methionine or other related compounds. We also show that SAM directly binds to and induces a conformational change in the leader RNA. Both binding of SAM and SAM-directed transcription termination were blocked by leader mutations that cause constitutive expression in vivo. Overproduction of SAM synthetase in Bacillus subtilis resulted in delay in induction of S box gene expression in response to methionine starvation, consistent with the hypothesis that SAM is the molecular effector in vivo. These results indicate that SAM concentration is sensed directly by the nascent transcript in the absence of a trans-acting factor.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

