A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle
- PMID: 12626751
- PMCID: PMC152252
- DOI: 10.1073/pnas.0538069100
A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle
Abstract
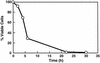
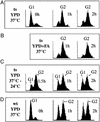
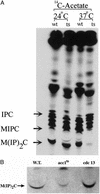
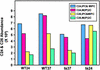
To elucidate the essential functions of acetyl-CoA carboxylase (ACC1FAS3) in Saccharomyces cerevisiae, a temperature-sensitive mutant (acc1(ts)) was constructed. When the acc1(ts) cells were synchronized in G(1) phase with alpha-factor at the permissive temperature of 24 degrees C and then released from the blockade and incubated at the restrictive temperature of 37 degrees C, 95% of the cell population became arrested at the G(2)M phase of the cell cycle despite the presence of fatty acids (C(14)-C(26)) in the medium. These cells developed large undivided nuclei, and the spindles of the arrested mutant cells were short. Shifting the G(2) arrested cells back to the permissive temperature resulted in a reversal of the cell-cycle arrest, with cells initiating mitosis. However, after 3 h of incubation at 37 degrees C, G(2) arrested mutant cells lost viability and displayed a uniquely altered nuclear envelope. Using [1-(14)C]acetate as a precursor for fatty acids synthesis, we identified the phospholipids and sphingolipids derived from acc1(ts) cells and wild-type cells at 24 degrees C and 37 degrees C, respectively. The levels of inositol-ceramides [IPC, MIPC, and M(IP)(2)C] and very long-chain fatty acids C(24) and C(26) declined sharply in the G(2)M arrested cells because of ACC inactivation. Shifting the acc1(ts) cells to 24 degrees C after 2 h of incubation at 37 degrees C resulted in reactivation of the ACC and elevation of the ceramides and very long-chain fatty acid syntheses with normal cell-cycle progression. In contrast, synthesis of wild-type inositol-ceramides, C(24) and C(26), fatty acids were elevated on incubation at 37 degrees C and declined when the cells shifted to the permissive temperature of 24 degrees C.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

