Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors
- PMID: 12626757
- PMCID: PMC152286
- DOI: 10.1073/pnas.0434590100
Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors
Abstract
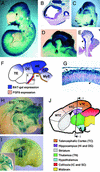
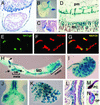
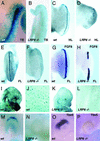
Wntbeta-catenin signaling plays key roles in several developmental and pathological processes. Domains of Wnt expression have been extensively investigated in the mouse, but the tissues receiving the signal remain largely unidentified. To define which cells respond to activated beta-catenin during mammalian development, we generated the beta-catenin-activated transgene driving expression of nuclear beta-galactosidase reporter (BAT-gal) transgenic mice, expressing the lacZ gene under the control of beta-cateninT cell factor responsive elements. Reporter gene activity is found in known organizing centers, such as the midhindbrain border and the limb apical ectodermal ridge. Moreover, BAT-gal expression identifies novel sites of Wnt signaling, like notochord, endothelia, and areas of the adult brain, revealing an unsuspected dynamic pattern of beta-catenin transcriptional activity. Expression of the transgene was analyzed in mutant backgrounds. In lipoprotein receptor-related protein 6-null homozygous mice, which lack a Wnt coreceptor, BAT-gal staining is absent in mutant tissues, indicating that BAT-gal mice are bona fide in vivo indicators of Wntbeta-catenin signaling. Analyses of BAT-gal expression in the adenomatous polyposis coli (multiple intestinal neoplasia+) background revealed betacatenin transcriptional activity in intestinal adenomas but surprisingly not in normal crypt cells. In summary, BAT-gal mice unveil the entire complexity of Wntbeta-catenin signaling in mammals and have broad application potentials for the identification of Wnt-responsive cell populations in development and disease.
Figures


References
-
- Bienz M, Clevers H. Cell. 2000;103:311–320. - PubMed
-
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Cell. 2001;105:533–545. - PubMed
-
- Wodarz A, Nusse R. Annu Rev Cell Dev Biol. 1998;14:59–88. - PubMed
-
- Niehrs C. Nature. 2001;413:787–788. - PubMed
-
- Peifer M, Polakis P. Science. 2000;287:1606–1609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

