Transcription factor YY1 functions as a PcG protein in vivo
- PMID: 12628927
- PMCID: PMC151054
- DOI: 10.1093/emboj/cdg124
Transcription factor YY1 functions as a PcG protein in vivo
Abstract
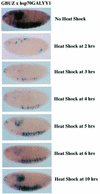
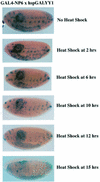
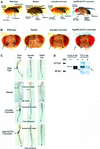
Polycomb group (PcG) proteins function as high molecular weight complexes that maintain transcriptional repression patterns during embryogenesis. The vertebrate DNA binding protein and transcriptional repressor, YY1, shows sequence homology with the Drosophila PcG protein, pleiohomeotic (PHO). YY1 might therefore be a vertebrate PcG protein. We used Drosophila embryo and larval/imaginal disc transcriptional repression systems to determine whether YY1 repressed transcription in a manner consistent with PcG function in vivo. YY1 repressed transcription in Drosophila, and this repression was stable on a PcG-responsive promoter, but not on a PcG-non-responsive promoter. PcG mutants ablated YY1 repression, and YY1 could substitute for PHO in repressing transcription in wing imaginal discs. YY1 functionally compensated for loss of PHO in pho mutant flies and partially corrected mutant phenotypes. Taken together, these results indicate that YY1 functions as a PcG protein. Finally, we found that YY1, as well as Polycomb, required the co-repressor protein CtBP for repression in vivo. These results provide a mechanism for recruitment of vertebrate PcG complexes to DNA and demonstrate new functions for YY1.
Figures



References
-
- Akasaka T., Kanno,M., Balling,R., Mieza,M.A., Taniguchi,M. and Koseki,H. (1996) A role for mel-18, a polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development, 122, 1513–1522. - PubMed
-
- Akasaka T. et al. (1997) The role of mel-18, a mammalian Polycomb group gene, during IL-7-dependent proliferation of lymphocyte precursors. Immunity, 7, 135–146. - PubMed
-
- Akasaka T. et al. (2001) Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development, 128, 1587–1597. - PubMed
-
- Alkema M.J., van der Lugt,N.M.T., Bobeldijk,R.C., Berns,A. and van Lohuizen,M. (1995) Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature, 374, 724–727. - PubMed
-
- Austen M., Luscher,B. and Luscher-Firzlaff,J.M. (1997) Characterization of the transcriptional regulator YY1. J. Biol. Chem., 272, 1709–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

