Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane
- PMID: 12629053
- PMCID: PMC2173757
- DOI: 10.1083/jcb.200212064
Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane
Abstract
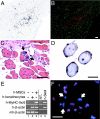
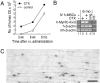
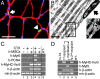
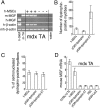
We have demonstrated previously that adult human synovial membrane-derived mesenchymal stem cells (hSM-MSCs) have myogenic potential in vitro (De Bari, C., F. Dell'Accio, P. Tylzanowski, and F.P. Luyten. 2001. Arthritis Rheum. 44:1928-1942). In the present study, we have characterized their myogenic differentiation in a nude mouse model of skeletal muscle regeneration and provide proof of principle of their potential use for muscle repair in the mdx mouse model of Duchenne muscular dystrophy. When implanted into regenerating nude mouse muscle, hSM-MSCs contributed to myofibers and to long term persisting functional satellite cells. No nuclear fusion hybrids were observed between donor human cells and host mouse muscle cells. Myogenic differentiation proceeded through a molecular cascade resembling embryonic muscle development. Differentiation was sensitive to environmental cues, since hSM-MSCs injected into the bloodstream engrafted in several tissues, but acquired the muscle phenotype only within skeletal muscle. When administered into dystrophic muscles of immunosuppressed mdx mice, hSM-MSCs restored sarcolemmal expression of dystrophin, reduced central nucleation, and rescued the expression of mouse mechano growth factor.
Figures


Comment in
-
Further proof of the plasticity of adult stem cells and their role in tissue repair.J Cell Biol. 2003 Mar 17;160(6):807-9. doi: 10.1083/jcb.200302117. J Cell Biol. 2003. PMID: 12642607 Free PMC article. Review.
References
-
- Bischoff, R. 1994. The satellite cell and muscle regeneration. Myogenesis. A.G. Engel and C. Franszini-Armstrong, editors. McGraw-Hill, New York. 97–118.
-
- Blau, H.M., G.K. Pavlath, E.C. Hardeman, C.P. Chiu, L. Silberstein, S.G. Webster, S.C. Miller, and C. Webster. 1985. Plasticity of the differentiated state. Science. 230:758–766. - PubMed
-
- Braun, S., C. Thioudellet, P. Rodriguez, D. Ali-Hadji, F. Perraud, N. Accart, J.M. Balloul, C. Halluard, B. Acres, B. Cavallini, and A. Pavirani. 2000. Immune rejection of human dystrophin following intramuscular injections of naked DNA in mdx mice. Gene Ther. 7:1447–1457. - PubMed
-
- Cooper, R.N., S. Tajbakhsh, V. Mouly, G. Cossu, M. Buckingham, and G.S. Butler-Browne. 1999. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 112:2895–2901. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

