Role of adhesin release for mucosal colonization by a bacterial pathogen
- PMID: 12629063
- PMCID: PMC2193847
- DOI: 10.1084/jem.20021153
Role of adhesin release for mucosal colonization by a bacterial pathogen
Abstract
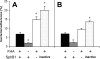
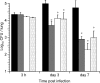
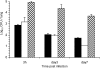
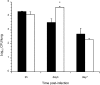
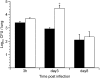
Pathogen attachment is a crucial early step in mucosal infections. This step is mediated by important virulence factors called adhesins. To exert these functions, adhesins are typically surface-exposed, although, surprisingly, some are also released into the extracellular milieu, the relevance of which has previously not been studied. To address the role of adhesin release in pathogenesis, we used Bordetella pertussis as a model, since its major adhesin, filamentous hemagglutinin (FHA), partitions between the bacterial surface and the extracellular milieu. FHA release depends on its maturation by the specific B. pertussis protease SphB1. We constructed SphB1-deficient mutants and found that they were strongly affected in their ability to colonize the mouse respiratory tract, although they adhered even better to host cells in vitro than their wild-type parent strain. The defect in colonization could be overcome by prior nasal instillation of purified FHA or by coinfection with FHA-releasing B. pertussis strains, but not with SphB1-producing FHA-deficient strains, ruling out a nonspecific effect of SphB1. These results indicate that the release of FHA is important for colonization, as it may facilitate the dispersal of bacteria from microcolonies and the binding to new sites in the respiratory tract.
Figures

References
-
- Beachey, E.H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachement of bacteria to mucosal surfaces. J. Infect. Dis. 143:325–345. - PubMed
-
- Isberg, R.R., and G. Tran Van Nhieu. 1994. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 2:10–14. - PubMed
-
- Wadström, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223–233. - PubMed
-
- Menozzi, F.D., K. Pethe, P. Bifani, F. Soncin, M.J. Brennan, and C. Locht. 2002. Enhanced bacterial virulence through exploitation of host glycosaminoglycans. Mol. Microbiol. 43:1379–1386. - PubMed

