Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus
- PMID: 12629163
- PMCID: PMC6741980
- DOI: 10.1523/JNEUROSCI.23-05-01593.2003
Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus
Abstract
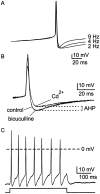
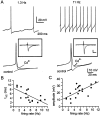
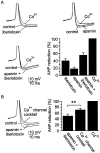
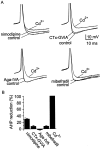
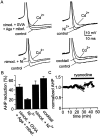
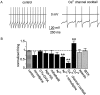
Cluster I neurons of the suprachiasmatic nucleus (SCN), which are thought to be pacemakers supporting circadian activity, fire spontaneous action potentials that are followed by a monophasic afterhyperpolarization (AHP). Using a brain slice preparation, we have found that the AHP has a shorter duration in cells firing at higher frequency, consistent with circadian modulation of the AHP. The AHP is supported by at least three subtypes of K(Ca) channels, including apamin-sensitive channels, iberiotoxin-sensitive channels, and channels that are insensitive to both of these antagonists. The latter K(Ca) channel subtype is involved in rate-dependent regulation of the AHP. Voltage-clamped, whole-cell Ca(2+) channel currents recorded from SCN neurons were dissected pharmacologically, revealing all of the major high-voltage activated subtypes: L-, N-, P/Q-, and R-type Ca(2+) channel currents. Application of Ca(2+) channel antagonists to spontaneously firing neurons indicated that predominantly L- and R-type currents trigger the AHP. Our findings suggest that apamin- and iberiotoxin-insensitive K(Ca) channels are subject to diurnal modulation by the circadian clock and that this modulation either directly or indirectly leads to the expression of a circadian rhythm in spiking frequency.
Figures


References
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. - PubMed
-
- Akasu T, Shoji S, Hasuo H. Inward rectifier and low-threshold calcium currents contribute to the spontaneous firing mechanism in neurons of the rat suprachiasmatic nucleus. Pflügers Arch. 1993;425:109–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
