The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons
- PMID: 12629169
- PMCID: PMC6741984
- DOI: 10.1523/JNEUROSCI.23-05-01649.2003
The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons
Abstract
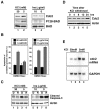
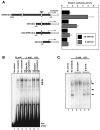
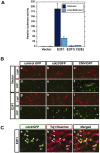
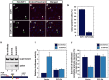
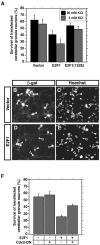
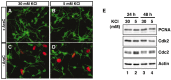
Neuronal apoptosis plays a critical role in the normal development of the mammalian brain and is thought to contribute to the pathogenesis of several neurologic disorders. However, the intracellular mechanisms underlying apoptosis of neurons remain incompletely understood. In the present study, we characterized a cell-cycle-based mechanism by which neuronal activity deprivation induces apoptosis of postmitotic neurons. Activity deprivation, but not growth factor withdrawal, was found to induce Cdc2 expression and consequent Cdc2-mediated apoptosis in granule neurons of the developing rat cerebellum. We found that activity deprivation induces cdc2 transcription in neurons via an E2F-binding element (EBE) within the cdc2 promoter. The transcription factor E2F1 that is expressed in granule neurons was found in DNA binding assays to bind to the EBE of the cdc2 gene. In chromatin immunoprecipitation analysis, endogenous E2F1 forms a complex with the promoter of the endogenous cdc2 gene in granule neurons, indicating that endogenous E2F1 is poised to activate transcription of the endogenous cdc2 gene in neurons. Consistent with this conclusion, a dominant interfering form of E2F, when expressed in granule neurons, blocked activity deprivation-induced cdc2 transcription. In other experiments, we found that the expression of E2F1 in granule neurons induces Cdc2 expression and promotes neuronal apoptosis via the activation of Cdc2. Remarkably, in contrast to inducing the E2F-mediated expression and activation of Cdc2 in granule neurons, activity deprivation fails to stimulate the expression of E2F-target genes that trigger DNA synthesis and replication. Together, our findings define a novel apoptotic mechanism whereby E2F selectively couples an activity deprivation-induced signal to cdc2 transcription in the absence of stimulating DNA synthesis and thus culminating in Cdc2-mediated apoptosis of postmitotic neurons.
Figures
Similar articles
-
Necdin downregulates CDC2 expression to attenuate neuronal apoptosis.J Neurosci. 2006 Nov 15;26(46):12003-13. doi: 10.1523/JNEUROSCI.3002-06.2006. J Neurosci. 2006. PMID: 17108174 Free PMC article.
-
E2Fs link the control of G1/S and G2/M transcription.EMBO J. 2004 Nov 24;23(23):4615-26. doi: 10.1038/sj.emboj.7600459. Epub 2004 Oct 28. EMBO J. 2004. PMID: 15510213 Free PMC article.
-
Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis.J Neurochem. 2003 Feb;84(4):814-28. doi: 10.1046/j.1471-4159.2003.01581.x. J Neurochem. 2003. PMID: 12562525
-
Cell cycle regulators in neural stem cells and postmitotic neurons.Neurosci Res. 2000 May;37(1):1-14. doi: 10.1016/s0168-0102(00)00101-2. Neurosci Res. 2000. PMID: 10802339 Review.
-
The cellular effects of E2F overexpression.Curr Top Microbiol Immunol. 1996;208:79-93. doi: 10.1007/978-3-642-79910-5_4. Curr Top Microbiol Immunol. 1996. PMID: 8575214 Review.
Cited by
-
Mechanistic insight into DNA damage and repair in ischemic stroke: exploiting the base excision repair pathway as a model of neuroprotection.Antioxid Redox Signal. 2011 May 15;14(10):1905-18. doi: 10.1089/ars.2010.3451. Epub 2010 Dec 2. Antioxid Redox Signal. 2011. PMID: 20677909 Free PMC article. Review.
-
Visualization of spatiotemporal energy dynamics of hippocampal neurons by mass spectrometry during a kainate-induced seizure.PLoS One. 2011 Mar 22;6(3):e17952. doi: 10.1371/journal.pone.0017952. PLoS One. 2011. PMID: 21445350 Free PMC article.
-
Targeting Huntington's disease through histone deacetylases.Clin Epigenetics. 2011 Aug;2(2):257-77. doi: 10.1007/s13148-011-0025-7. Epub 2011 Feb 18. Clin Epigenetics. 2011. PMID: 22704341 Free PMC article.
-
Class IIA HDACs in the regulation of neurodegeneration.Front Biosci. 2008 Jan 1;13:1072-82. doi: 10.2741/2745. Front Biosci. 2008. PMID: 17981613 Free PMC article. Review.
-
Role of the transcription factor E2F1 in CXCR4-mediated neurotoxicity and HIV neuropathology.Neurobiol Dis. 2007 Jan;25(1):17-26. doi: 10.1016/j.nbd.2006.08.004. Epub 2006 Sep 28. Neurobiol Dis. 2007. PMID: 17011204 Free PMC article.
References
-
- Altman J, Bayer S. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC; New York: 1997.
-
- Bonni A, Brunet A, West A, Datta SR, Takasu M, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
