A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity
- PMID: 12629174
- PMCID: PMC6741950
- DOI: 10.1523/JNEUROSCI.23-05-01697.2003
A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity
Abstract
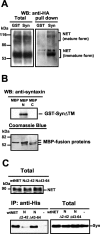
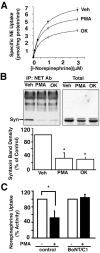
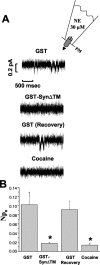
Norepinephrine (NE) transporters (NETs) terminate noradrenergic synaptic transmission and represent a major therapeutic target for antidepressant medications. NETs and related transporters are under intrinsic regulation by receptor and kinase-linked pathways, and clarification of these pathways may suggest candidates for the development of novel therapeutic approaches. Syntaxin 1A, a presynaptic soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein, interacts with NET and modulates NET intrinsic activity. NETs colocalize with and bind to syntaxin 1A in both native preparations and heterologous systems. Protein kinase C activation disrupts surface NET/syntaxin 1A interactions and downregulates NET activity in a syntaxin-dependent manner. Syntaxin 1A binds the NH(2) terminal domain of NET, and a deletion of this domain both eliminates NET/syntaxin 1A associations and prevents phorbol ester-triggered NET downregulation. Whereas syntaxin 1A supports the surface trafficking of NET proteins, its direct interaction with NET limits transporter catalytic function. These two contradictory roles of syntaxin 1A on NET appear to be linked and reveal a dynamic cycle of interactions that allow for the coordinated control between NE release and reuptake.
Figures



Similar articles
-
Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A.J Neurosci. 2002 May 1;22(9):3342-51. doi: 10.1523/JNEUROSCI.22-09-03342.2002. J Neurosci. 2002. PMID: 11978810 Free PMC article.
-
Syntaxin 1A up-regulates GABA transporter expression by subcellular redistribution.Mol Membr Biol. 2001 Jan-Mar;18(1):39-44. Mol Membr Biol. 2001. PMID: 11396610
-
Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A.Mol Cell Neurosci. 2007 Feb;34(2):251-60. doi: 10.1016/j.mcn.2006.11.007. Epub 2006 Dec 26. Mol Cell Neurosci. 2007. PMID: 17188889 Free PMC article.
-
The role of SNARE proteins in trafficking and function of neurotransmitter transporters.Handb Exp Pharmacol. 2006;(175):181-96. doi: 10.1007/3-540-29784-7_9. Handb Exp Pharmacol. 2006. PMID: 16722236 Review.
-
How the monoamine transporter garden grows.Mol Pharmacol. 2005 Aug;68(2):272-4. doi: 10.1124/mol.105.014951. Epub 2005 May 23. Mol Pharmacol. 2005. PMID: 15911691 Review.
Cited by
-
Differentiation of rodent behavioral phenotypes and methylphenidate action in sustained and flexible attention tasks.Brain Res. 2016 Jun 15;1641(Pt B):306-19. doi: 10.1016/j.brainres.2015.11.039. Epub 2015 Dec 10. Brain Res. 2016. PMID: 26688113 Free PMC article.
-
Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A.Neuropharmacology. 2008 Oct;55(5):763-70. doi: 10.1016/j.neuropharm.2008.06.018. Epub 2008 Jun 18. Neuropharmacology. 2008. PMID: 18602929 Free PMC article.
-
Synthesis and in silico evaluation of novel compounds for PET-based investigations of the norepinephrine transporter.Molecules. 2015 Jan 20;20(1):1712-30. doi: 10.3390/molecules20011712. Molecules. 2015. PMID: 25608857 Free PMC article.
-
Analysis of knock-out mice to determine the role of HPC-1/syntaxin 1A in expressing synaptic plasticity.J Neurosci. 2006 May 24;26(21):5767-76. doi: 10.1523/JNEUROSCI.0289-06.2006. J Neurosci. 2006. PMID: 16723534 Free PMC article.
-
Recognition of psychostimulants, antidepressants, and other inhibitors of synaptic neurotransmitter uptake by the plasma membrane monoamine transporters.AAPS J. 2005 Oct 27;7(3):E739-51. doi: 10.1208/aapsj070374. AAPS J. 2005. PMID: 16353950 Free PMC article. Review.
References
-
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport. I. Protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998a;287:733–743. - PubMed
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport. II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998b;287:744–751. - PubMed
-
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. - PubMed
-
- Arnsten AF. Modulation of prefrontal cortical–striatal circuits: relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol. 2001;85:333–341. - PubMed
-
- Axelrod J, Kopin IJ. The uptake, storage, release, and metabolism of noradrenaline in sympathetic nerves. Prog Brain Res. 1969;31:21–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
