Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus
- PMID: 12629188
- PMCID: PMC6741948
- DOI: 10.1523/JNEUROSCI.23-05-01840.2003
Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus
Abstract
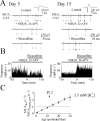
In the developing rat hippocampus, ictal epileptiform activity can be elicited easily in vitro during the first three postnatal weeks. Changes in neuronal ion transport during this time cause the effects of GABA(A) receptor (GABA(A)-R) activation to shift gradually from strongly depolarizing to hyperpolarizing. It is not known whether the depolarizing effects of GABA and the propensity for ictal activity are causally linked. A key question is whether the GABA-mediated depolarization is excitatory, which we defined operationally as being sufficient to trigger action potentials. We assessed the effect of endogenous GABA on ictal activity and neuronal firing rate in hippocampal slices from postnatal day 1 (P1) to P30. In extracellular recordings, there was a strong correlation between the postnatal age at which GABA(A)-R antagonists decreased action potential frequency (P23) and the age at which ictal activity could be induced by elevated potassium (P23). In addition, there was a strong correlation between the fraction of slices in which ictal activity was induced by elevated potassium concentrations and the fractional decrease in action potential firing when GABA(A)-Rs were blocked in the presence of ionotropic glutamate receptor antagonists. Finally, ictal activity induced by elevated potassium was blocked by the GABA(A)-R antagonists bicuculline and SR-95531 (gabazine) and increased in frequency and duration by GABA(A)-R agonists isoguvacine and muscimol. Thus, the propensity of the developing hippocampus for ictal activity is highly correlated with the effect of GABA on action potential probability and reversed by GABA(A) antagonists, indicating that GABA-mediated excitation is causally linked to ictal activity in this developmental window.
Figures






Similar articles
-
Anticonvulsant action of GABA in the high potassium-low magnesium model of ictogenesis in the neonatal rat hippocampus in vivo and in vitro.J Neurophysiol. 2005 Oct;94(4):2987-92. doi: 10.1152/jn.00138.2005. Epub 2005 Jul 6. J Neurophysiol. 2005. PMID: 16000527
-
Shunting and hyperpolarizing GABAergic inhibition in the high-potassium model of ictogenesis in the developing rat hippocampus.Hippocampus. 2007;17(3):210-9. doi: 10.1002/hipo.20259. Hippocampus. 2007. PMID: 17294460
-
Ictal epileptiform activity in the CA3 region of hippocampal slices produced by pilocarpine.J Neurophysiol. 1998 Jun;79(6):3019-29. doi: 10.1152/jn.1998.79.6.3019. J Neurophysiol. 1998. PMID: 9636105
-
The depolarizing action of GABA controls early network activity in the developing hippocampus.Mol Neurobiol. 2011 Apr;43(2):97-106. doi: 10.1007/s12035-010-8147-z. Epub 2010 Nov 3. Mol Neurobiol. 2011. PMID: 21042953 Review.
-
Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions?J Physiol. 2013 Feb 15;591(4):765-74. doi: 10.1113/jphysiol.2012.237958. Epub 2012 Aug 13. J Physiol. 2013. PMID: 22890709 Free PMC article. Review.
Cited by
-
Molecular mechanisms of antiseizure drug activity at GABAA receptors.Seizure. 2013 Oct;22(8):589-600. doi: 10.1016/j.seizure.2013.04.015. Epub 2013 May 14. Seizure. 2013. PMID: 23683707 Free PMC article. Review.
-
NR1 knockdown reveals CA1 injury during a developmental period of high seizure susceptibility despite reduced seizure activity.Neuromolecular Med. 2007;9(4):298-314. doi: 10.1007/s12017-007-8009-7. Epub 2007 Aug 14. Neuromolecular Med. 2007. PMID: 17999204
-
Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis.PLoS Comput Biol. 2011 Sep;7(9):e1002149. doi: 10.1371/journal.pcbi.1002149. Epub 2011 Sep 8. PLoS Comput Biol. 2011. PMID: 21931544 Free PMC article.
-
Compromised maturation of GABAergic inhibition underlies abnormal network activity in the hippocampus of epileptic Ca2+ channel mutant mice, tottering.Pflugers Arch. 2015 Apr;467(4):737-52. doi: 10.1007/s00424-014-1555-6. Epub 2014 Jun 20. Pflugers Arch. 2015. PMID: 24947601
-
Sex Differences in the Development of the Rodent Corticolimbic System.Front Neurosci. 2020 Sep 30;14:583477. doi: 10.3389/fnins.2020.583477. eCollection 2020. Front Neurosci. 2020. PMID: 33100964 Free PMC article. Review.
References
-
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–134. - PubMed
-
- Ben Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated “menage a trois.”. Trends Neurosci. 1997;20:523–529. - PubMed
-
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
