Localized lesion of caudal part of lobus parolfactorius caused impulsive choice in the domestic chick: evolutionarily conserved function of ventral striatum
- PMID: 12629194
- PMCID: PMC6741993
- DOI: 10.1523/JNEUROSCI.23-05-01894.2003
Localized lesion of caudal part of lobus parolfactorius caused impulsive choice in the domestic chick: evolutionarily conserved function of ventral striatum
Abstract
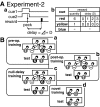
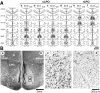
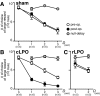
Effects of bilateral chemical lesions of the medial basal ganglia [lobus parolfactorius (LPO)] were examined in 7- to 14-d-old domestic chicks. Chicks were trained in a color discrimination task, in which the subject had to peck one of the two colored beads associated with rewards that differed in quantity (amount of food) and/or temporal proximity (delay of food delivery from peck). In experiment 1, food was given without delay, and chicks successfully learned to choose a colored bead that was associated with a larger reward than the other. In experiment 2, a colored bead (red) was associated with a large reward delivered after a delay (D = 1, 2, or 3 sec), whereas another (yellow) was associated with a small reward delivered immediately. In intact and sham-operated conditions, chicks with a longer D chose the red bead progressively fewer times. Selective lesions to the caudal LPO (but not the rostral LPO) caused impulsive choice, and the ablated chicks chose the yellow bead and gained a small-immediate reward regardless of D. However, when retrained in a null-delay condition (D = 0 sec), the lesioned chick chose the red bead again. Ability to associate novel colors with reward was also unimpaired. These results suggest that the LPO may be responsible for the anticipation of reward proximity and involved in a suppression of impulsiveness by which animals seek immediate gains. The present results also indicate a striking similarity in functional roles between the avian LPO and the nucleus accumbens/ventral striatum in mammals.
Figures


References
-
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. - PubMed
-
- Alcock J. Animal behavior: an evolutionary approach, Ed 7. Sinauer; Sunderland, MA: 2001.
-
- Aoki N, Izawa E-I, Naito J, Matsushima T. Representation of memorized color in the intermediate ventral archistriatum (amygdala homolog) of domestic chicks. Soc Neurosci Abstr. 2002;28:189.13.
-
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
