On the role of nitric oxide in hippocampal long-term potentiation
- PMID: 12629199
- PMCID: PMC6741944
- DOI: 10.1523/JNEUROSCI.23-05-01941.2003
On the role of nitric oxide in hippocampal long-term potentiation
Abstract
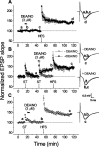
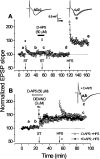
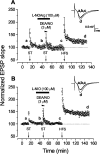
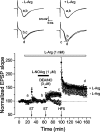
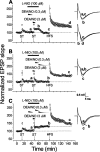
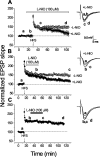
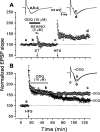
Nitric oxide (NO) functions in several types of synaptic plasticity, including hippocampal long-term potentiation (LTP), in which it may serve as a retrograde messenger after postsynaptic NMDA receptor activation. In accordance with a prediction of this hypothesis, and with previous findings using guinea pig tissue, exogenous NO, when paired with a short tetanus (ST) to afferent fibers, generated a stable NMDA receptor-independent potentiation of rat CA1 hippocampal synaptic transmission that occluded LTP. Contrary to predictions, however, the pairing-induced potentiation was abolished in the presence of NO synthase inhibitors, indicating that endogenous NO is required for exogenous NO to facilitate LTP. Periodic application of NO while endogenous NO synthesis was blocked indicated that a tonic low level is necessary on both sides of the NO-ST pairing for the plasticity to occur. A similar dependence on tonic NO seems to extend to LTP, because application of an NO synthase inhibitor 5 min after tetanic stimulation blocked LTP as effectively as adding it beforehand. The posttetanus time window during which NO operated was restricted to <15 min. Inhibition of the guanylyl cyclase-coupled NO receptor indicated that the potentiation resulting from NO-ST pairing and the NO signal transduction pathway during early LTP are both through cGMP. We conclude that NO does not function simply as an acute signaling molecule in LTP induction but has an equally important role outside this phase. The results resonate with observations concerning the role of the hippocampal NO-cGMP pathway in certain types of learning behavior.
Figures
Similar articles
-
Exogenous nitric oxide causes potentiation of hippocampal synaptic transmission during low-frequency stimulation via the endogenous nitric oxide-cGMP pathway.Eur J Neurosci. 2001 Aug;14(4):585-94. doi: 10.1046/j.0953-816x.2001.01680.x. Eur J Neurosci. 2001. PMID: 11556884
-
Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase.J Neurosci. 2002 Dec 1;22(23):10116-22. doi: 10.1523/JNEUROSCI.22-23-10116.2002. J Neurosci. 2002. PMID: 12451112 Free PMC article.
-
Nitric oxide signalling is required for the generation of anoxia-induced long-term potentiation in the hippocampus.Eur J Neurosci. 1997 Oct;9(10):2202-6. doi: 10.1111/j.1460-9568.1997.tb01387.x. Eur J Neurosci. 1997. PMID: 9421180
-
Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia.Neurochem Int. 2004 Nov;45(6):895-901. doi: 10.1016/j.neuint.2004.03.020. Neurochem Int. 2004. PMID: 15312984 Review.
-
Nitric oxide and carbon monoxide as possible retrograde messengers in hippocampal long-term potentiation.J Neurobiol. 1994 Jun;25(6):652-65. doi: 10.1002/neu.480250607. J Neurobiol. 1994. PMID: 8071665 Review.
Cited by
-
Impact of environmental thermal stimulation on activation of hypothalamic neuronal nitric oxide synthase during the prenatal ontogenesis in Muscovy ducks.ScientificWorldJournal. 2012;2012:416936. doi: 10.1100/2012/416936. Epub 2012 Apr 19. ScientificWorldJournal. 2012. PMID: 22611339 Free PMC article.
-
Heterocyclic N-Oxides - An Emerging Class of Therapeutic Agents.Curr Med Chem. 2015;22(24):2819-57. doi: 10.2174/0929867322666150619104007. Curr Med Chem. 2015. PMID: 26087764 Free PMC article. Review.
-
Hippocampal GABAergic synapses possess the molecular machinery for retrograde nitric oxide signaling.J Neurosci. 2007 Jul 25;27(30):8101-11. doi: 10.1523/JNEUROSCI.1912-07.2007. J Neurosci. 2007. PMID: 17652601 Free PMC article.
-
Conjugated polymer-based fluorescence turn-on sensor for nitric oxide.Org Lett. 2005 Aug 4;7(16):3573-5. doi: 10.1021/ol0513903. Org Lett. 2005. PMID: 16048345 Free PMC article.
-
Pharmacological manipulation of cGMP and NO/cGMP in CNS drug discovery.Nitric Oxide. 2019 Jan 1;82:59-74. doi: 10.1016/j.niox.2018.10.006. Epub 2018 Oct 28. Nitric Oxide. 2019. PMID: 30394348 Free PMC article. Review.
References
-
- Baratti CM, Kopf SR. A nitric oxide synthase inhibitor impairs memory storage in mice. Neurobiol Learn Mem. 1996;65:197–201. - PubMed
-
- Bernabeu R, de Stein ML, Fin C, Izquierdo I, Medina JH. Role of hippocampal NO in the acquisition and consolidation of inhibitory avoidance learning. NeuroReport. 1995;6:1498–1500. - PubMed
-
- Bernabeu R, Schmitz P, Faillace MP, Izquierdo I, Medina JH. Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. NeuroReport. 1996;7:585–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
