Enhanced insulin sensitivity in mice lacking ganglioside GM3
- PMID: 12629211
- PMCID: PMC152312
- DOI: 10.1073/pnas.0635898100
Enhanced insulin sensitivity in mice lacking ganglioside GM3
Abstract
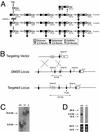
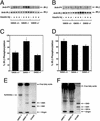
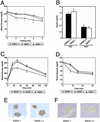
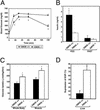
Gangliosides are sialic acid-containing glycosphingolipids that are present on all mammalian plasma membranes where they participate in recognition and signaling activities. We have established mutant mice that lack GM3 synthase (CMP-NeuAc:lactosylceramide alpha2,3-sialyltransferase; EC 2.4.99.-). These mutant mice were unable to synthesize GM3 ganglioside, a simple and widely distributed glycosphingolipid. The mutant mice were viable and appeared without major abnormalities but showed a heightened sensitivity to insulin. A basis for the increased insulin sensitivity in the mutant mice was found to be enhanced insulin receptor phosphorylation in skeletal muscle. Importantly, the mutant mice were protected from high-fat diet-induced insulin resistance. Our results show that GM3 ganglioside is a negative regulator of insulin signaling, making it a potential therapeutic target in type 2 diabetes.
Figures

Similar articles
-
Ganglioside depletion and EGF responses of human GM3 synthase-deficient fibroblasts.Glycobiology. 2008 Aug;18(8):593-601. doi: 10.1093/glycob/cwn039. Epub 2008 May 14. Glycobiology. 2008. PMID: 18480157
-
Ganglioside GM3 promotes cell migration by regulating MAPK and c-Fos/AP-1.Oncogene. 2006 Jun 29;25(28):3948-55. doi: 10.1038/sj.onc.1209416. Epub 2006 Feb 20. Oncogene. 2006. PMID: 16491123
-
Peroxynitrite mediates muscle insulin resistance in mice via nitration of IRbeta/IRS-1 and Akt.Toxicol Appl Pharmacol. 2009 Nov 15;241(1):101-10. doi: 10.1016/j.taap.2009.08.005. Epub 2009 Aug 12. Toxicol Appl Pharmacol. 2009. PMID: 19682478
-
Glycosphingolipids and insulin resistance.Prog Lipid Res. 2009 May-Jul;48(3-4):196-205. doi: 10.1016/j.plipres.2009.03.002. Epub 2009 Mar 20. Prog Lipid Res. 2009. PMID: 19303901 Review.
-
Ganglioside GM3 as a gatekeeper of obesity-associated insulin resistance: Evidence and mechanisms.FEBS Lett. 2015 Oct 24;589(21):3221-7. doi: 10.1016/j.febslet.2015.09.018. Epub 2015 Oct 8. FEBS Lett. 2015. PMID: 26434718 Review.
Cited by
-
DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases.J Radiat Res. 2021 Jul 10;62(4):564-573. doi: 10.1093/jrr/rrab032. J Radiat Res. 2021. PMID: 33912932 Free PMC article. Review.
-
Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet.Int J Obes (Lond). 2013 Aug;37(8):1064-70. doi: 10.1038/ijo.2012.191. Epub 2012 Dec 4. Int J Obes (Lond). 2013. PMID: 23207405 Free PMC article.
-
Sialylation regulates brain structure and function.FASEB J. 2015 Jul;29(7):3040-53. doi: 10.1096/fj.15-270983. Epub 2015 Apr 6. FASEB J. 2015. PMID: 25846372 Free PMC article.
-
Gangliosides of the Vertebrate Nervous System.J Mol Biol. 2016 Aug 14;428(16):3325-3336. doi: 10.1016/j.jmb.2016.05.020. Epub 2016 May 31. J Mol Biol. 2016. PMID: 27261254 Free PMC article. Review.
-
Mice lacking sialyltransferase ST3Gal-II develop late-onset obesity and insulin resistance.Glycobiology. 2017 Jan;27(2):129-139. doi: 10.1093/glycob/cww098. Epub 2016 Sep 28. Glycobiology. 2017. PMID: 27683310 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

