Control of neurotransmitter release by an internal gel matrix in synaptic vesicles
- PMID: 12629223
- PMCID: PMC152319
- DOI: 10.1073/pnas.0336914100
Control of neurotransmitter release by an internal gel matrix in synaptic vesicles
Abstract
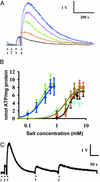
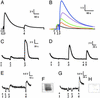
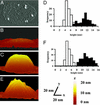
Neurotransmitters are stored in synaptic vesicles, where they have been assumed to be in free solution. Here we report that in Torpedo synaptic vesicles, only 5% of the total acetylcholine (ACh) or ATP content is free, and that the rest is adsorbed to an intravesicular proteoglycan matrix. This matrix, which controls ACh and ATP release by an ion-exchange mechanism, behaves like a smart gel. That is, it releases neurotransmitter and changes its volume when challenged with small ionic concentration change. Immunodetection analysis revealed that the synaptic vesicle proteoglycan SV2 is the core of the intravesicular matrix and is responsible for immobilization and release of ACh and ATP. We suggest that in the early steps of vesicle fusion, this internal matrix regulates the availability of free diffusible ACh and ATP, and thus serves to modulate the quantity of transmitter released.
Figures


Similar articles
-
Acetylcholine changes underlying transmission of a single nerve impulse in the presence of 4-aminopyridine in Torpedo.J Physiol. 1982 Apr;325:461-79. doi: 10.1113/jphysiol.1982.sp014162. J Physiol. 1982. PMID: 6286942 Free PMC article.
-
Cholinergic vesicle specific proteoglycan: stability in isolated vesicles and in synaptosomes during induced transmitter release.J Neurochem. 1988 Jan;50(1):11-6. doi: 10.1111/j.1471-4159.1988.tb13223.x. J Neurochem. 1988. PMID: 3121784
-
Acetylcholine, ATP, and proteoglycan are common to synaptic vesicles isolated from the electric organs of electric eel and electric catfish as well as from rat diaphragm.J Neurochem. 1986 Nov;47(5):1449-62. doi: 10.1111/j.1471-4159.1986.tb00778.x. J Neurochem. 1986. PMID: 3760871
-
Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction.Prog Neurobiol. 2003 Nov;71(4):269-303. doi: 10.1016/j.pneurobio.2003.10.003. Prog Neurobiol. 2003. PMID: 14698765 Review.
-
Quantal acetylcholine release: vesicle fusion or intramembrane particles?Microsc Res Tech. 2000 Apr 1;49(1):38-46. doi: 10.1002/(SICI)1097-0029(20000401)49:1<38::AID-JEMT5>3.0.CO;2-0. Microsc Res Tech. 2000. PMID: 10757877 Review.
Cited by
-
Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels.Sci Rep. 2018 Apr 10;8(1):5726. doi: 10.1038/s41598-018-23877-3. Sci Rep. 2018. PMID: 29636491 Free PMC article.
-
Presynaptic K(+) channels, vesicular Ca(2+)/H (+) antiport--synaptotagmin, and acetylcholinesterase, three mechanisms cutting short the cholinergic signal at neuromuscular and nerve-electroplaque junctions.J Mol Neurosci. 2014 Jul;53(3):377-86. doi: 10.1007/s12031-013-0212-4. Epub 2014 Jan 4. J Mol Neurosci. 2014. PMID: 24390960
-
Alzheimer's disease protein Abeta1-42 does not disrupt isolated synaptic vesicles.Biochim Biophys Acta. 2008 May;1782(5):326-34. doi: 10.1016/j.bbadis.2008.02.002. Epub 2008 Feb 20. Biochim Biophys Acta. 2008. PMID: 18339328 Free PMC article.
-
Synaptic vesicle protein 2 enhances release probability at quiescent synapses.J Neurosci. 2006 Jan 25;26(4):1303-13. doi: 10.1523/JNEUROSCI.2699-05.2006. J Neurosci. 2006. PMID: 16436618 Free PMC article.
-
Large structural change in isolated synaptic vesicles upon loading with neurotransmitter.Biophys J. 2009 Nov 4;97(9):2577-84. doi: 10.1016/j.bpj.2009.08.032. Biophys J. 2009. PMID: 19883601 Free PMC article.
References
-
- Fernandez-Chacón R, Sudhof T C. Annu Rev Physiol. 1999;61:753–776. - PubMed
-
- Jahn R, Sudhof T C. Annu Rev Biochem. 1999;68:863–911. - PubMed
-
- Álvarez de Toledo G, Fernández-Chacón R, Fernández J M. Nature. 1993;363:554–558. - PubMed
-
- Albillos A, Dernick G, Horstmann H, Almers W, Álvarez de Toledo G, Lindau M. Nature. 1997;389:509–512. - PubMed
-
- Monck J R, Fernandez J M. Curr Opin Cell Biol. 1996;8:524–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

