Unraveling monogenic channelopathies and their implications for complex polygenic disease
- PMID: 12629596
- PMCID: PMC1180344
- DOI: 10.1086/374317
Unraveling monogenic channelopathies and their implications for complex polygenic disease
Abstract
Ion channels are a large family of >400 related proteins representing >1% of our genetic endowment; however, ion-channel diseases reflect a relatively new category of inborn error. They were first recognized in 1989, with the discovery of cystic fibrosis transmembrane conductance regulator, and rapidly advanced as positional and functional studies converged in the dissection of components of the action potential of excitable tissues. Although it remains true that diseases of excitable tissue still most clearly illustrate this family of disease, ion-channel disorders now cover the gamut of medical disciplines, causing significant pathology in virtually every organ system, producing a surprising range of often unanticipated symptoms, and providing valuable targets for pharmacological intervention. Many of the features shared among the monogenic ion-channel diseases provide a general framework for formulating a foundation for considering their intrinsically promising role in polygenic disease. Since an increasingly important approach to the identification of genes underlying polygenic disease is to identify "functional candidates" within a critical region and to test their disease association, it becomes increasingly important to appreciate how these ion-channel mechanisms can be implicated in pathophysiology.
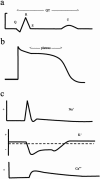
Figures

References
Electronic-Database Information
-
- GeneCards, http://bioinfo.weizmann.ac.il/cards/index.html (for database of human genes, their products, and their involvement in diseases)
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CFTR, RW, JLN, LQT1, LQT2, LQT5, LQT6, LQT3, Brugada syndrome, Andersen syndrome, LQT7, DFNA2, SIDS, SCCMS, HOKPP, MHS5, MHS1, Becker myotonia, Thomsen myotonia, HYPP, MSH2, paramyotonia congenita, potassium activated myotonia, benign familial neonatal convulsions BFNC1, benign familial neonatal convulsions BFNC2, KCNQ5, ADNFLE type 1, ADNFLE type 3, GEFS+, SCN1B, SCN1A, SCN2A, SMEI, GABRG2, JME, CACNB4, episodic ataxia type 2, FHM1, SCA6, and episodic ataxia type 1)
References
-
- Abbott GW, Butler MH, Bendahhou S, Dalakas MC, Ptacek LJ, Goldstein SAN (2001) MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell 104:217–231 - PubMed
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97:175–187 - PubMed
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M (2001) Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci 21:6095–6104 - PMC - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G (1996) K(v)LQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384:78–80 - PubMed
-
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme J-F, Baulac M, Brice A, Bruzzone R, LeGuern E (2001) First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma-2-subunit gene. Nat Genet 28:46–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

