COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus
- PMID: 12631664
- PMCID: PMC1773580
- DOI: 10.1136/gut.52.4.527
COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus
Abstract
Background and aims: Cyclooxygenase 2 (COX-2) and prostaglandins (PGs) participate in the pathogenesis of inflammatory postoperative ileus. We sought to determine whether the emerging neuronal modulator COX-2 plays a significant role in primary afferent activation during postoperative ileus using spinal Fos expression as a marker.
Methods: Rats, and COX-2(+/+) and COX-2(-/-) mice underwent simple intestinal manipulation. The effect of intestinal manipulation on Fos immunoreactivity (IR) in the L(5)-S(1) spinal cord, in situ circumference, and postoperative leucocytic infiltrate of the intestinal muscularis was measured. Postoperative PGE(2) production was measured in peritoneal lavage fluid. The dependence of these parameters on COX-2 was studied in pharmacological (DFU, Merck- Frosst, selective COX-2 inhibitor) and genetic (COX-2(-/-) mice) models.
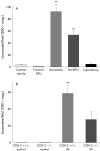
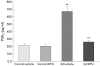
Results: Postoperative Fos IR increased 3.7-fold in rats and 2.2-fold in mice. Both muscularis leucocytic infiltrate and the circumference of the muscularis increased significantly in rats and COX-2(+/+) mice postoperatively, indicating dilating ileus. Surgical manipulation markedly increased PGE(2) levels in the peritoneal cavity. DFU pretreatment and the genetic absence of COX-2(-/-) prevented dilating ileus, and leucocytic infiltrate was diminished by 40% with DFU and by 54% in COX-2(-/-) mice. DFU reversed postsurgical intra- abdominal PGE(2) levels to normal. Fos IR after intestinal manipulation was attenuated by approximately 50% in DFU treated rats and in COX-2(-/-) mice.
Conclusions: Postoperatively, small bowel manipulation causes a significant and prolonged increase in spinal Fos expression, suggesting prolonged primary afferent activation. COX-2 plays a key role in this response. This activation of primary afferents may subsequently initiate inhibitory motor reflexes to the gut, contributing to postoperative ileus.
Figures






Similar articles
-
Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus.Gastroenterology. 2001 Dec;121(6):1354-71. doi: 10.1053/gast.2001.29605. Gastroenterology. 2001. PMID: 11729115
-
Differential sensitization of afferent neuronal pathways during postoperative ileus in the mouse jejunum.Ann Surg. 2008 May;247(5):791-802. doi: 10.1097/SLA.0b013e31816a9d97. Ann Surg. 2008. PMID: 18438116
-
Differential salutary effects of nonselective and selective COX-2 inhibitors in postoperative ileus in rats.J Surg Res. 2003 Feb;109(2):161-9. doi: 10.1016/s0022-4804(02)00095-1. J Surg Res. 2003. PMID: 12643859
-
Colonic postoperative inflammatory ileus in the rat.Ann Surg. 2002 Jul;236(1):56-66. doi: 10.1097/00000658-200207000-00010. Ann Surg. 2002. PMID: 12131086 Free PMC article.
-
Prostaglandins and cyclooxygenases [correction of cycloxygenases] in the spinal cord.Prog Neurobiol. 2001 Jul;64(4):327-63. doi: 10.1016/s0301-0082(00)00063-0. Prog Neurobiol. 2001. PMID: 11275357 Review.
Cited by
-
Behavioral and physiological characterization of PKC-dependent phosphorylation in the Grin2a∆PKC mouse.Brain Res. 2016 Sep 1;1646:315-326. doi: 10.1016/j.brainres.2016.06.022. Epub 2016 Jun 15. Brain Res. 2016. PMID: 27317637 Free PMC article.
-
Controlling postoperative ileus by vagal activation.World J Gastroenterol. 2010 Apr 14;16(14):1683-7. doi: 10.3748/wjg.v16.i14.1683. World J Gastroenterol. 2010. PMID: 20379998 Free PMC article. Review.
-
The ICAM-1 antisense oligonucleotide ISIS-3082 prevents the development of postoperative ileus in mice.Br J Pharmacol. 2005 Sep;146(2):252-8. doi: 10.1038/sj.bjp.0706303. Br J Pharmacol. 2005. PMID: 15997238 Free PMC article.
-
Electroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammation.Sci Rep. 2017 Jan 4;7:39801. doi: 10.1038/srep39801. Sci Rep. 2017. PMID: 28051128 Free PMC article.
-
Muscularis macrophages: Key players in intestinal homeostasis and disease.Cell Immunol. 2018 Aug;330:142-150. doi: 10.1016/j.cellimm.2017.12.009. Epub 2017 Dec 26. Cell Immunol. 2018. PMID: 29291892 Free PMC article. Review.
References
-
- Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000;87:1480–93. - PubMed
-
- Holzer P, Lippe IT, Holzer-Petsche U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology 1986;91:360–3. - PubMed
-
- Zittel TT, Meile T, Jehle EC, et al. Intraperitoneal capsaicin treatment reduces postoperative gastric ileus in awake rats. Langenbecks Arch Surg 2001;386:204–11. - PubMed
-
- Zittel TT, Lloyd KC, Rothenhofer I, et al. Calcitonin gene-related peptide and spinal afferents partly mediate postoperative colonic ileus in the rat. Surgery 1998;123:518–27. - PubMed
-
- Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology 2000;118:316–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
